
Hot Rolling Steel
Forming Technology and Heat Treatments
There are various methods of
forming steel
into finished products, including
hot forging
, hot and cold rolling, seamless tube making and welded tube making. The most widely used process is hot rolling, which accounts for over 90% of all steel production.
Rolling is a forming process, which causes permanent change of shape (set) by plastic deformation of the material as it passes between sets of steel rolls.Sets of cylindrical rolls reduce the cross-sectional thickness of the metal whilst simultaneously causing it to become elongated. Other rolling processes are employed to change the cross-sectional shape of the metal using shaped rolls while other methods form the metal into a specific shape for a particular purpose.

Steelmaking Production
Steelmaking Technology
Production efficiency has been improved by increasing the melt capacity of furnaces, implementing on-line computer control modules, and introducing new technologies, such as the combined blowing process for LD (Linz Donawitz) converters, the Ultra High Power (UHP) electric furnace, the ladle steelmaking processes and continuous casting.
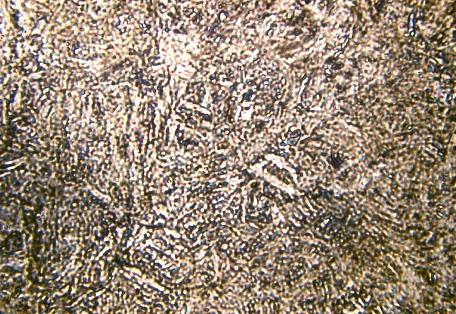
Microstructure of Metals
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25x magnification.The microstructure of a material (which can be broadly classified into metallic, polymeric, ceramic and composite) can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behavior, wear resistance, and so on, which in turn govern the application of these materials in industrial practice.
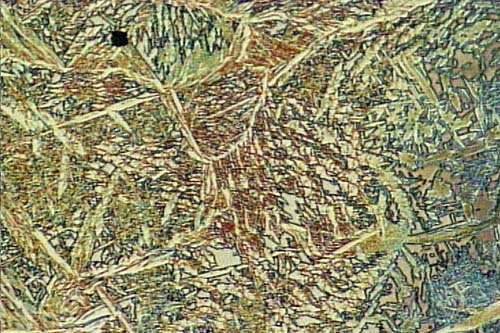
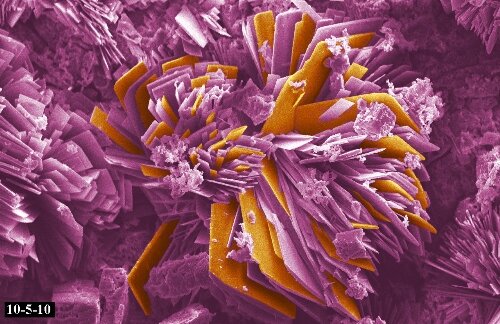
Microstructure of a weld used in duplex stainless steel, 2205, 250X original magnification. A color mixture of austenite, ferrite and sigma phases.
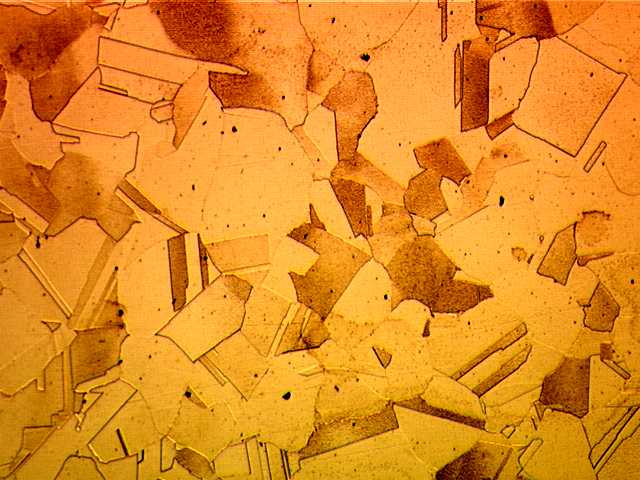
Microstructure of Austenitic Stainless Steel
Advanced Ceramics
 A ceramic
is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous (e.g., a glass). Because most common ceramics are crystalline, the definition of ceramic is often restricted to inorganic crystalline materials, as opposed to the noncrystalline glasses.
A ceramic
is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous (e.g., a glass). Because most common ceramics are crystalline, the definition of ceramic is often restricted to inorganic crystalline materials, as opposed to the noncrystalline glasses.
The earliest ceramics were pottery objects made from clay, either by itself or mixed with other materials, hardened in fire. Later ceramics were glazed and fired to create a colored, smooth surface. Ceramics now include domestic, industrial and building products and art objects. In the 20th century, new ceramic materials were developed for use in advanced ceramic engineering; for example, in semiconductors.













 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope