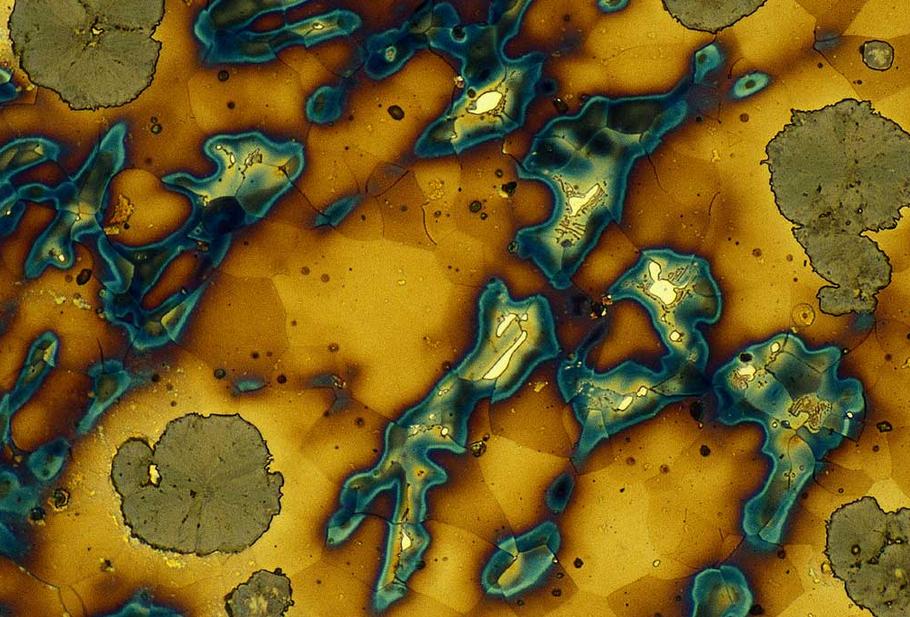
What is Graphite ?
Graphite is very soft. It is used in the “lead” of pencils, which contain no lead at all, but are made of graphite and clay. Strangely enough, graphite has exactly the same chemical formula as diamond, the hardest substance known to mankind. They are both carbon. In diamond, the atoms of carbon lock together into an incredibly strong structure. In graphite, the atoms are arranged in a different way, in layers. This means that one layer can be rubbed off quite easily, which is what happens when you write with a pencil. If you tried to write with a diamond pencil, you would gouge holes in the paper, and the table underneath!
 Graphite
Graphite
The mineral graphite is an allotrope of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek “to draw/write”, for its use in pencils, where it is commonly called lead (not to be confused with the metallic element lead). Unlike diamond (another carbon allotrope), graphite is an electrical conductor, a semimetal.
You might also like
| Types of Cast Iron Cast Iron - a Definition Cast irons typically... | What is Malleable Cast Iron ? Malleable Cast Iron - a Definition Malleable... | Do you know Meehanite metal ? Meehanite Meehanite is a trademark for... | What is Grey Cast Iron ? Grey Cast Iron - Meaning and Definition Grey... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope