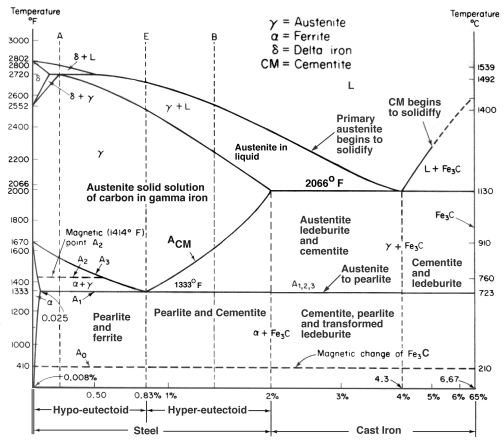
Cast irons typically contain 2-4 wt% of carbon with a high silicon concentrations and a greater concentration of impurities than steels. The carbon equivalent (CE) of a cast iron helps to distinguish the grey irons which cool into a microstructure containing graphite and and the white irons where the carbon is present mainly as cementite. The carbon equivalent is defined as:
A high cooling rate and a low carbon equivalent favours the formation of white cast iron whereas a low cooling rate or a high carbon equivalent promotes grey cast iron.
 FeC Phasae Diagram
FeC Phasae Diagram
During solidification, the major proportion of the carbon precipitates in the form of graphite or cementite. When solidification is just complete, the precipitated phase is embedded in a matrix of austenite which has an equilibrium carbon concentration of about 2 wt%. On further cooling, the carbon concentration of the austenite decreases as more cementite or graphite precipitates from solid solution. For conventional cast irons, the austenite then decomposes into pearlite at the eutectoid temperature. However, in grey cast irons, if the cooling rate through the eutectoid temperature is sufficiently slow, then a completely ferritic matrix is obtained with the excess carbon being deposited on the already existing graphite. White cast irons are hard and brittle, they cannot easily be machined.
You might also like
| Cast Iron Cast iron is derived from pig iron,... | Austempered Ductile Iron (ADI) Austempered Ductile Iron (ADI) is... | Structure and Components of Steel The engineering properties of steel,... | Metallurgy Glossary Metallurgy Glossary Activity: A function... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope