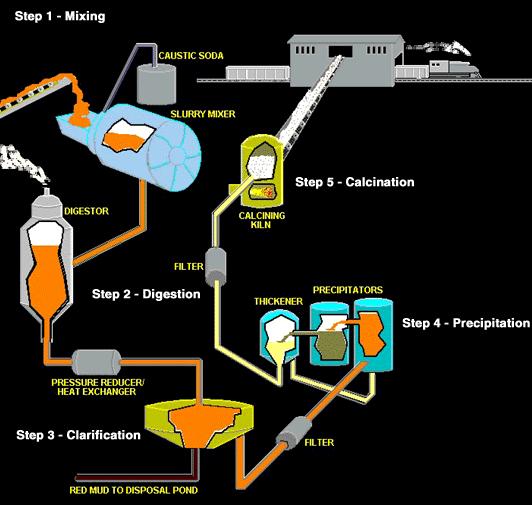
Alumina is a white granular material, a little finer than table salt, and is properly called aluminum oxide. The Bayer refining process used by alumina refineries worldwide involves four steps - digestion, clarification, precipitation and calcination. The aluminum oxide is dissolved by the caustic soda, then precipitated out of this solution, washed, and heated to drive off water.
 Alumina Balls
Alumina Balls
What’s left is the white powder called alumina, which is transformed into aluminum metal in the smelting process. Aluminum originates as an oxide called alumina. Because aluminum itself does not occur in nature as a metal, the processing of aluminum took a giant leap forward with the advent of electricity. Deposits of bauxite ore are mined and refined into alumina—one of the feedstocks for aluminum metal. Then alumina and electricity are combined in a cell with molten electrolyte called cryolite. Direct-current electricity is passed from a consumable carbon anode into the cryolite, splitting the aluminum oxide into molten aluminum metal and carbon-dioxide.
Aluminium oxide is an amphoteric oxide with the chemical formula Al2O3. It is commonly referred to as alumina (alpha-alumina), or corundum in its crystalline form, as well as many other names, reflecting its widespread occurrence in nature and industry. Its most significant use is in the production of aluminium metal, although it is also used as an abrasive owing to its hardness and as a refractory material owing to its high melting point. There is also a cubic ?-alumina with important technical applications.
Alumina Production
Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colors to trace impurities. Rubies are given their characteristic deep red color and their laser qualities by traces of chromium. Sapphires come in different colors given by various other impurities, such as iron and titanium.
Alumina Powder
Aluminium oxide is an electrical insulator but has a relatively high thermal conductivity for a ceramic material. In its most commonly occurring crystalline form, called corundum or alpha-aluminium oxide, its hardness makes it suitable for use as an abrasive and as a component in cutting tools.
Aluminium oxide is responsible for the resistance of metallic aluminium to weathering. Metallic aluminium is very reactive with atmospheric oxygen, and a thin passivation layer of alumina (4 nm thickness) forms in about 100 picoseconds on any exposed aluminium surface. This layer protects the metal from further oxidation.
The thickness and properties of this oxide layer can be enhanced using a process called anodising. A number of alloys, such as aluminium bronzes, exploit this property by including a proportion of aluminium in the alloy to enhance corrosion resistance. The alumina generated by anodising is typically amorphous, but discharge assisted oxidation processes such as plasma electrolytic oxidation result in a significant proportion of crystalline alumina in the coating, enhancing its hardness.
You might also like
| How Aluminum is Produced ? Aluminum Production ? Aluminum manufacture... | The Bayer and Hall-Heroult Process What is The Bayer and Hall-Heroult Process... | Aluminum alloys What is Aluminum Alloys ? Aluminium alloys... | Application of Titanium Dioxide What is Titanium Dioxide ? Titanium dioxide,... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope