Aluminum Oxide Overview
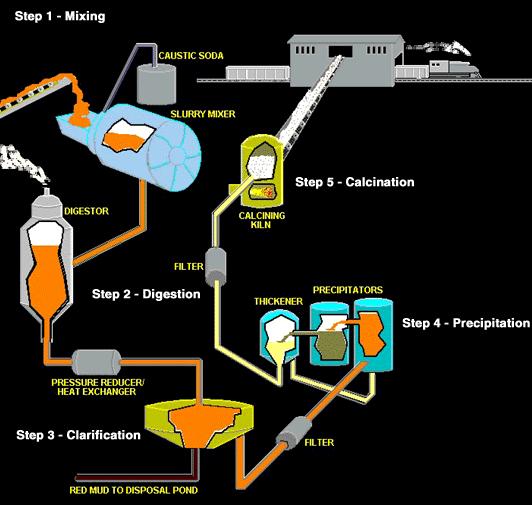
Alumina is a white granular material, a little finer than table salt, and is properly called aluminum oxide. The Bayer refining process used by alumina refineries worldwide involves four steps - digestion, clarification, precipitation and calcination. The aluminum oxide is dissolved by the caustic soda, then precipitated out of this solution, washed, and heated to drive off water.
 Alumina Balls
Alumina Balls
What’s left is the white powder called alumina, which is transformed into aluminum metal in the smelting process. Aluminum originates as an oxide called alumina. Because aluminum itself does not occur in nature as a metal, the processing of aluminum took a giant leap forward with the advent of electricity. Deposits of bauxite ore are mined and refined into alumina—one of the feedstocks for aluminum metal. Then alumina and electricity are combined in a cell with molten electrolyte called cryolite. Direct-current electricity is passed from a consumable carbon anode into the cryolite, splitting the aluminum oxide into molten aluminum metal and carbon-dioxide.
You might also like
| The Bayer and Hall-Heroult Process What is The Bayer and Hall-Heroult Process... | How Aluminum is Produced ? Aluminum Production ? Aluminum manufacture... | Application of Titanium Dioxide What is Titanium Dioxide ? Titanium dioxide,... | Aluminum Welding - Beginner Guide to Weld Aluminum How to weld Aluminum ? Aluminum is the most... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope