Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. Metallurgy is distinguished from the craft of metalworking. The science and technology of metals and alloys. Process metallurgy is concerned with the extraction of metals from their ores and with refining of metals, physical metallurgy, with the physical and mechanical properties of metals as affected by composition, processing, and environmental conditions and mechanical metallurgy, with the response of metals to applied forces.
 Metal Casting
Metal Casting
In its general, modern sense, metallurgy is the science that studies the chemical and physical properties of metals, including how they perform when used for culturally useful industrial purposes. The term often refers to the procedures used in extracting metals from ore, as well as to the processes related to metals purification and alloy production. Copper, tin, silver, and meteoric iron, which was used by the Egyptians to make weapons, all underwent some form of metalworking process in various ancient cultures.
The first evidence of a standard metallurgy technology appeared during the Bronze Age, which started around 3,500 BC, when it was discovered that by heating and combining copper and tin, a bronze alloy could be created. The Iron Age began around 1,200 BC when the Hittites discovered how to extract iron from ore and work it to advance their cultural aims. Georg Agricola, considered to be the father of metallurgy, detailed ore mining and metal extraction procedures, as well as other aspects of the science, in his 16th century book, De re metallica.
 Aluminum Casting Process
Aluminum Casting Process
The word was originally (1593) an alchemist’s term for the extraction of metals from minerals: the ending -urgy signifying a process, especially manufacturing: it was in this sense it was used by the 1797 Encyclopaedia Britannica. In the late 19th century it was extended to the more general scientific study of metals and alloys and related processes.
The extraction of iron from its ore into a workable metal is much more difficult. This includes the ancient and medieval kingdoms and empires of the Middle East and Near East, ancient Iran, ancient Egypt, ancient Nubia, and Anatolia (Turkey), Ancient Nok, Carthage, the Greeks and Romans of ancient Europe, medieval Europe, ancient and medieval China, ancient and medieval India, ancient and medieval Japan, amongst others. A 16th century book by Georg Agricola called De re metallica describes the highly developed and complex processes of mining metal ores, metal extraction and metallurgy of the time.
 Casting Mould
Casting Mould
Extractive metallurgy is the practice of removing valuable metals from an ore and refining the extracted raw metals into a purer form. In order to convert a metal oxide or sulfide to a purer metal, the ore must be reduced physically, chemically, or electrolytically. Extractive metallurgists are interested in three primary streams : feed, concentrate (valuable metal oxide/sulfide), and tailings (waste). Concentrating the particles of value in a form supporting separation enables the desired metal to be removed from waste products.
 Crucible
Crucible
The solution is collected and processed to extract valuable metals. Ore bodies often contain more than one valuable metal. Additionally, a concentrate may contain more than one valuable metal. That concentrate would then be processed to separate the valuable metals into individual constituents.
 Hot Rolled Metal
Hot Rolled Metal
Common engineering metals include aluminium, chromium, copper, iron, magnesium, nickel, titanium and zinc. Much effort has been placed on understanding the iron-carbon alloy system, which includes steels and cast irons. Cast irons, including ductile iron are also part of the iron-carbon system.
 Hot Rolled MetalCoil
Hot Rolled MetalCoil
Stainless steel or galvanized steel are used where resistance to corrosion is important. Aluminium alloys and magnesium alloys are used for applications where strength and lightness are required. Copper-nickel alloys (such as Monel) are used in highly corrosive environments and for non-magnetic applications. For extremely high temperatures, single crystal alloys are used to minimize creep.
 Metal Forging
Metal Forging
This involves the production of alloys, the shaping, the heat treatment and the surface treatment of the product. The task of the metallurgist is to achieve balance between material properties such as cost, weight, strength, toughness, hardness, corrosion, fatigue resistance, and performance in temperature extremes. In a saltwater environment, ferrous metals and some aluminium alloys corrode quickly. Metals under continual cyclic loading can suffer from metal fatigue. Metals under constant stress at elevated temperatures can creep.
 Hot Forging Process
Hot Forging Process
Metals can be heat treated to alter the properties of strength, ductility, toughness, hardness or resistance to corrosion. Common heat treatment processes include annealing, precipitation strengthening, quenching, and tempering. The annealing process softens the metal by heating it and then allowing it to cool very slowly, which gets rid of stresses in the metal and makes the grain structure large and soft-edged so that when the metal is hit or stressed it dents or perhaps bends, rather than breaking, it is also easier to sand, grind, or cut annealed metal.
 Underwater Welding
Underwater Welding
Quenching is the process of cooling a high-carbon steel very quickly after you have heated it, thus “freezing” the steel’s molecules in the very hard martensite form, which makes the metal harder. Tempering relieves stresses in the metal that were caused by the hardening process; tempering makes the metal less hard while making it better able to sustain impacts without breaking. Crystallography allows identification of unknown materials and reveals the crystal structure of the sample.
You might also like
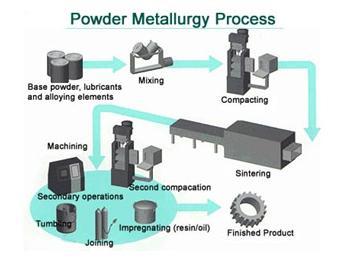
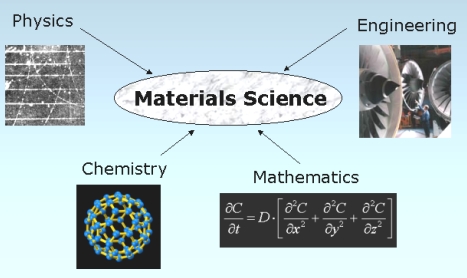
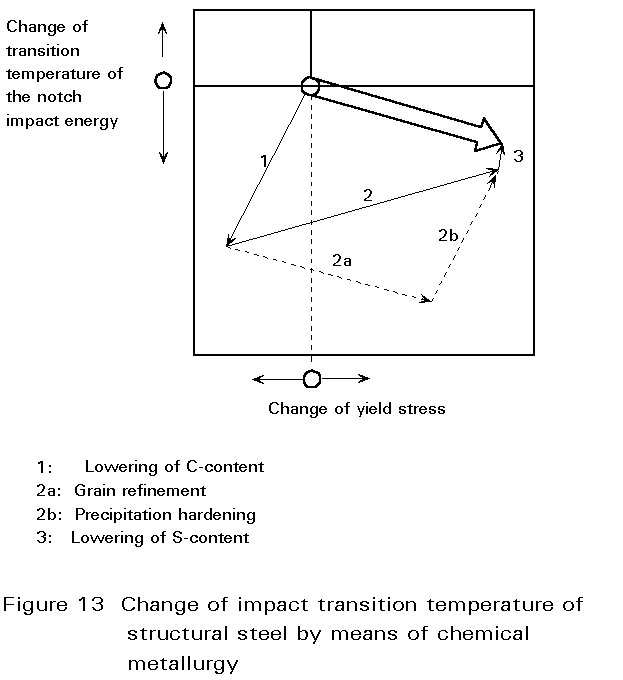
| Powder Metallurgy Powder Metallurgy Process, source : https://www.themetalcasting.com/ Powder... | Materials Science - Overview Materials can be natural - like wood, or... | Optimal Combination of STRENGTH and TOUGHNESS Preceding sections have described the influence... | Metallurgy Glossary Metallurgy Glossary Activity: A function... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope