Nanoparticles are particles that have one dimension that is 100 nanometers or less in size. The properties of many conventional materials change when formed from nanoparticles. This is typically because nanoparticles have a greater surface area per weight than larger particles, this causes them to be more reactive to certain other molecules.
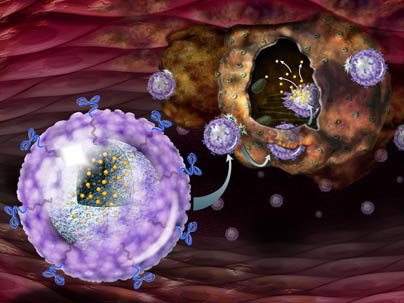 Nanoparticle
Nanoparticle
Nanoparticles are used, or being evaluated for use, in many fields. The list below introduces many of the uses under development. You can use the links in each paragraph to go to a detailed explanation. Particles are further classified according to size : in terms of diameter, coarse particles cover a range between 10,000 and 2,500 nanometers. Fine particles are sized between 2,500 and 100 nanometers. Ultrafine particles, ornanoparticles are sized between 100 and 1 nanometers. Nanoparticles may or may not exhibit size-related properties that differ significantly from those observed in fine particles or bulk materials.Nanopowders are agglomerates of ultrafine particles, nanoparticles, or nanoclusters. Nanometer-sized single crystals, or single-domain ultrafine particles, are often referred to as nanocrystals.
TEM (a, b, and c) images of prepared mesoporous silica nanoparticles with mean outer diameter : (a) 20nm, (b) 45nm, and (c) 80nm. The insets are a high magnification of mesoporous silica particle. The luster originated within the film itself, which contained silver and copper nanoparticles dispersed homogeneously in the glassy matrix of the ceramic glaze.
The chemical processing and synthesis of high performance technological components for the private, industrial and military sectors requires the use of high purity ceramics, polymers, glass-ceramics and material composites. In condensed bodies formed from fine powders, the irregular particle sizes and shapes in a typical powder often lead to non-uniform packing morphologies that result in packing density variations in the powder compact.
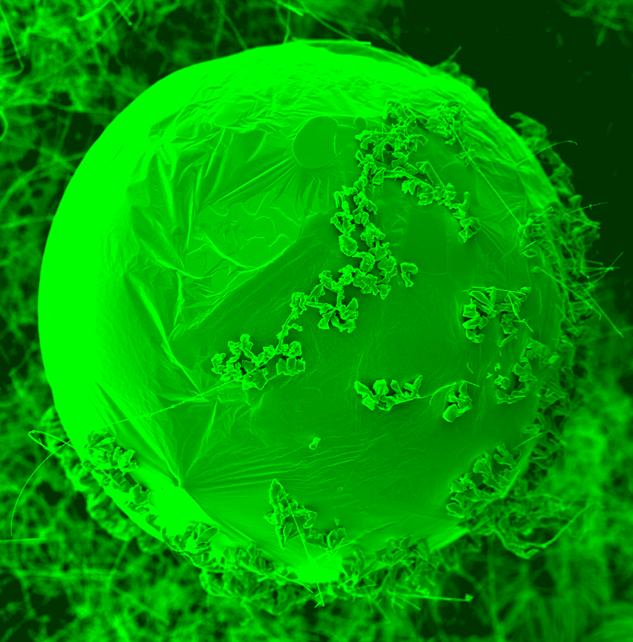 Nanoparticles are sized between 100 and 1 nanometers
Nanoparticles are sized between 100 and 1 nanometers
Inert gas evaporation and inert gas deposition are free many of these defects due to the distillation (c.f. purification) nature of the process and having enough time to form single crystal particles, however even their non-aggreated deposits have lognormal size distribution, which is typical with nanoparticles. However, even in this case, random residence times in the growth zone, due to the combination of drift and diffusion, result in a size distribution appearing lognormal.
Monodisperse nanoparticles and colloids provide this potential. Nanoparticles are of great scientific interest as they are effectively a bridge between bulk materials and atomic or molecularstructures. A bulk material should have constant physical properties regardless of its size, but at the nano-scale size-dependent properties are often observed. Thus, the properties of materials change as their size approaches the nanoscale and as the percentage of atoms at the surface of a material becomes significant. The interesting and sometimes unexpected properties of nanoparticles are therefore largely due to the large surface area of the material, which dominates the contributions made by the small bulk of the material.
Nanoparticles often possess unexpected optical properties as they are small enough to confine their electrons and produce quantum effects. For example gold nanoparticles appear deep red to black in solution. Nanoparticles of usually yellow gold and gray silicon are red in color. Gold nanoparticles melt at much lower temperatures (~300 °C for 2.5 nm size) than the gold slabs (1064 °C).
Other size-dependent property changes include quantum confinement in semiconductor particles, surface plasmon resonance in some metal particles and superparamagnetism in magnetic materials. Suspensions of nanoparticles are possible since the interaction of the particle surface with the solvent is strong enough to overcome density differences, which otherwise usually result in a material either sinking or floating in a liquid.
The high surface area to volume ratio of nanoparticles provides a tremendous driving force for diffusion, especially at elevated temperatures. Sintering can take place at lower temperatures, over shorter time scales than for larger particles. For example the presence of titanium dioxide nanoparticles imparts what we call the self-cleaning effect, and the size being nano-range, the particles can not be observed. Zinc oxide particles have been found to have superior UV blocking properties compared to its bulk substitute. Clay nanoparticles when incorporated into polymer matrices increase reinforcement, leading to stronger plastics, verifiable by a higher glass transition temperature and other mechanical property tests.
These nanoparticles are hard, and impart their properties to the polymer (plastic). Metal, dielectric, and semiconductor nanoparticles have been formed, as well as hybrid structures (e.g., core-shell nanoparticles). Nanoparticles made of semiconducting material may also be labeled quantum dots if they are small enough (typically sub 10 nm) that quantization of electronic energy levels occurs. Semi-solid and soft nanoparticles have been manufactured. A prototype nanoparticle of semi-solid nature is the liposome.
Nanoparticles with one half hydrophilic and the other half hydrophobic are termed Janus particles and are particularly effective for stabilizing emulsions. There are several methods for creating nanoparticles, including both attrition and pyrolysis. In attrition, macro or micro scale particles are ground in a ball mill, a planetary ball mill, or other size reducing mechanism. The resulting particles are air classified to recover nanoparticles. The resulting solid (a version of soot) is air classified to recover oxide particles from by-product gases. Pyrolysis often results in aggregates and agglomerates rather than single primary particles.
A thermal plasma can also deliver the energy necessary to cause evaporation of small micrometer size particles. Nanoparticles are formed upon cooling while exiting the plasma region. The main types of the thermal plasma torches used to produce nanoparticles are dc plasma jet, dc arc plasma and radio frequency (RF) induction plasmas. The resulting mixture of plasma gas and silica vapour can be rapidly cooled by quenching with oxygen, thus ensuring the quality of the fumed silica produced.
The RF plasma method has been used to synthesize different nanoparticle materials, for example synthesis of various ceramic nanoparticles such as oxides, carbours/carbides and nitrides of Ti and Si (see Induction plasma technology). Inert-gas condensation is frequently used to make nanoparticles from metals with low melting points. The supercooled metal vapor condenses into nanometer-sized particles, which can be entrained in the inert gas stream and deposited on a substrate or studied in situ.
The sol-gel process is a wet-chemical technique (also known as chemical solution deposition) widely used recently in the fields of materials science and ceramic engineering. Typical precursors are metal alkoxides and metal chlorides, which undergo hydrolysis and polycondensation reactions to form either a network “elastic solid” or a colloidal suspension (ordispersion) – a system composed of discrete (often amorphous) submicrometer particles dispersed to various degrees in a host fluid. Formation of a metal oxide involves connecting the metal centers with oxo (M-O-M) or hydroxo (M-OH-M) bridges, therefore generating metal-oxo or metal-hydroxo polymers in solution.Sol-gel derived materials have diverse applications in optics, electronics, energy, space, (bio)sensors, medicine (e.g. controlled drug release) and separation (e.g. chromatography) technology
The term colloid is used primarily to describe a broad range of solid–liquid (and/or liquid–liquid) mixtures, all of which contain distinct solid (and/or liquid) particles which are dispersed to various degrees in a liquid medium. The term is specific to the size of the individual particles, which are larger than atomic dimensions but small enough to exhibit Brownian motion. This critical size range (or particle diameter) typically ranges from nanometers (0.000000001 m) to micrometers (0.000001 m). At the small end of the size range, nanoparticles are often referred to as clusters. The study of fine particles is called micromeritics.
Nanoparticle characterization is necessary to establish understanding and control of nanoparticle synthesis and applications. Common techniques areelectron microscopy (TEM, SEM), atomic force microscopy (AFM), dynamic light scattering (DLS), x-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF), ultraviolet-visible spectroscopy, dual polarisation interferometry and nuclear magnetic resonance (NMR). For biological applications, the surface coating should be polar to give high aqueous solubility and prevent nanoparticle aggregation. Monovalent nanoparticles, bearing a single binding site, avoid clustering and so are preferable for tracking the behavior of individual proteins.
Nanoparticles present possible dangers, both medically and environmentally. A recent study looking at the effects of ZnO nanoparticles on human immune cells has found varying levels of susceptibility to cytotoxicity. Previously activated memory T cells were more susceptible than naive T cells, implying a relationship between cell-cycle and nanoparticle susceptibility. Diesel nanoparticles have been found to damage the cardiovascular system in a mouse model.Concern has also been raised over the health effects of respirable nanoparticles from certain combustion processes.







 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope