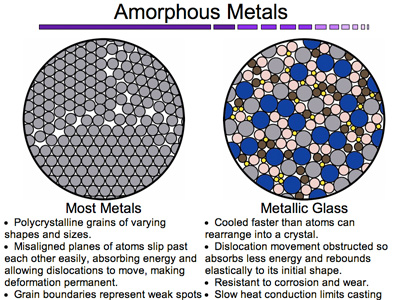
An amorphous metal is a metal with a disordered atomic structure, in contrast to most metals, which have a regular structure. These substances are also called metallic glasses, because one way of making amorphous metals resembles the procedure for making glass, but using metal instead of silica. Studies indicate that amorphous metals may be more than twice as strong as normal metal, and are ideal for military armor, weighing the same as ordinary metal. Because of the material’s disordered structure, it is also more resistant to corrosion and wear.
 Amorphous metals
Amorphous metals
Amorphous metals were first created at Caltech by Pol Duwez in 1957. Duwez created the amorphous metal by cooling an alloy (Au80Si20) from a liquid state in under a fraction of a second. The cooling rate had to exceed a million degrees Kelvin per second, so cooling the metal from the liquid state to a solid state had to occur in milliseconds. Cooling this quickly prevented the metal from crystallizing like a typical metal, giving it its unique amorphous structure. At the beginning, the forms of amorphous metal were limited, consisting mostly of thin ribbons, foils, and wires. These constraints were enforced by the necessity of a fast cooling rate.
Amorphous metals, also called metallic glasses, consist of metal with a disordered atomic structure. They can be twice as strong as steel. Because of their disordered structure, they can disperse impact energy more effectively than a metal crystal, which has points of weakness. Amorphous metals are made by quickly cooling molten metal before it has a chance to align itself in a crystal pattern. Amorphous metals may the military’s next generation of armor, before they adopt diamondoid armor in mid-century. On the green side of things, amorphous metals have electronic properties that improve the efficiency of power grids by as much as 40%, saving us thousands of tons of fossil fuel emissions.
An amorphous metal is a metal with a disordered atomic structure, in contrast to most metals
An amorphous metal is a metallic material with a disordered atomic-scale structure. In contrast to most metals, which are crystalline and therefore have a highly ordered arrangement of atoms, amorphous alloys are non-crystalline. Materials in which such a disordered structure is produced directly from the liquid state during cooling are called “glasses”, and so amorphous metals are commonly referred to as “metallic glasses” or “glassy metals”. However, there are several ways besides extremely rapid cooling in which amorphous metals can be produced, including physical vapor deposition, solid-state reaction, ion irradiation, melt spinning, and mechanical alloying. Some scientists do not consider amorphous metals produced by these techniques to be glasses. However, materials scientists commonly consider amorphous alloys to be a single class of materials, regardless of how they are prepared.
An amorphous metal is a metallic material with a disordered atomic-scale structure
In the past, small batches of amorphous metals have been produced through a variety of quick-cooling methods. For instance, amorphous metal wires have been produced by sputtering molten metal onto a spinning metal disk. The rapid cooling, on the order of millions of degrees a second, is too fast for crystals to form and the material is “locked in” a glassy state. More recently a number of alloys with critical cooling rates low enough to allow formation of amorphous structure in thick layers (over 1 millimeter) had been produced; these are known as bulk metallic glasses (BMG). Liquidmetal sells a number of titanium-based BMGs, developed in studies originally carried out at Caltech. More recently, batches of amorphous steel have been produced that demonstrate strengths much greater than conventional steel alloys.
On top of one base is a disk of a metal alloy with an amorphous atomic structure. That one causes the bearing to bounce. And bounce. And bounce. The amorphous structure doesn’t allow much energy transfer from the ball bearings.
The first reported metallic glass was an alloy (Au75Si25) produced at Caltech by W. Klement (Jr.), Willens and Duwez in 1960. This and other early glass-forming alloys had to be cooled extremely rapidly (on the order of one megakelvin per second, 106 K/s) to avoid crystallization. An important consequence of this was that metallic glasses could only be produced in a limited number of forms (typically ribbons, foils, or wires) in which one dimension was small so that heat could be extracted quickly enough to achieve the necessary cooling rate. As a result, metallic glass specimens (with a few exceptions) were limited to thicknesses of less than one hundred micrometers.
In 1969, an alloy of 77.5% palladium, 6% copper, and 16.5% silicon was found to have critical cooling rate between 100 to 1000 K/s. In 1976, H. Liebermann and C. Graham developed a new method of manufacturing thin ribbons of amorphous metal on a supercooled fast-spinning wheel. This was an alloy of iron, nickel, phosphorus and boron. The material, known as Metglas, was commercialized in early 1980s and used for low-loss power distribution transformers (Amorphous metal transformer). Metglas-2605 is composed of 80% iron and 20% boron, has Curie temperature of 373 °C and a room temperature saturation magnetization of 1.56 teslas.
In the early 1980s, glassy ingots with 5 mm diameter were produced from the alloy of 55% palladium, 22.5% lead, and 22.5% antimony, by surface etching followed with heating-cooling cycles. Using boron oxide flux, the achievable thickness was increased to a centimeter.The research in Tohoku University and Caltech yielded multicomponent alloys based on lanthanum, magnesium, zirconium, palladium, iron, copper, and titanium, with critical cooling rate between 1 K/s to 100 K/s, comparable to oxide glasses.In 1988, alloys of lanthanum, aluminium, and copper ore were found to be highly glass-forming.
Amorphous metal core transformers
In the 1990s, however, new alloys were developed that form glasses at cooling rates as low as one kelvin per second. These cooling rates can be achieved by simple casting into metallic molds. These “bulk” amorphous alloys can be cast into parts of up to several centimeters in thickness (the maximum thickness depending on the alloy) while retaining an amorphous structure. The best glass-forming alloys are based on zirconium and palladium, but alloys based on iron, titanium, copper, magnesium, and other metals are also known. Many amorphous alloys are formed by exploiting a phenomenon called the “confusion” effect. Such alloys contain so many different elements (often a dozen or more) that upon cooling at sufficiently fast rates, the constituent atoms simply cannot coordinate themselves into the equilibrium crystalline state before their mobility is stopped. In this way, the random disordered state of the atoms is “locked in”.
In 1992, the first commercial amorphous alloy, Vitreloy 1 (41.2% Zr, 13.8% Ti, 12.5% Cu, 10% Ni, and 22.5% Be), was developed at Caltech, as a part of Department of Energy and NASA research of new aerospace materials. More variants followed.In 2004, two groups succeeded in producing bulk amorphous steel, one at Oak Ridge National Laboratory, the other at University of Virginia. The Oak Ridge group refers to their product as “glassy steel”. The product is non-magnetic at room temperature and significantly stronger than conventional steel, though a long research and development process remains before the introduction of the material into public or military use.
Amorphous metal is usually an alloy rather than a pure metal. The alloys contain atoms of significantly different sizes, leading to low free volume (and therefore up to orders of magnitude higher viscosity than other metals and alloys) in molten state. The viscosity prevents the atoms moving enough to form an ordered lattice. The material structure also results in low shrinkage during cooling, and resistance to plastic deformation. The absence of grain boundaries, the weak spots of crystalline materials, leads to better resistance to wear and corrosion. Amorphous metals, while technically glasses, are also much tougher and less brittle than oxide glasses and ceramics. Thermal conductivity of amorphous materials is lower than of crystals. As formation of amorphous structure relies on fast cooling, this limits the maximum achievable thickness of amorphous structures.
To achieve formation of amorphous structure even during slower cooling, the alloy has to be made of three or more components, leading to complex crystal units with higher potential energy and lower chance of formation. The atomic radius of the components has to be significantly different (over 12%), to achieve high packing density and low free volume. The combination of components should have negative heat of mixing, inhibiting crystal nucleation and prolongs the time the molten metal stays in supercooled state. The alloys of boron, silicon, phosphorus, and other glass formers with magnetic metals (iron, cobalt, nickel) are magnetic, with low coercivity and high electrical resistance. The high resistance leads to low losses by eddy currents when subjected to alternating magnetic fields, a property useful for e.g. transformer magnetic cores.
Amorphous alloys have a variety of potentially useful properties. In particular, they tend to be stronger than crystalline alloys of similar chemical composition, and they can sustain larger reversible (“elastic”) deformations than crystalline alloys. Amorphous metals derive their strength directly from their non-crystalline structure, which does not have any of the defects (such as dislocations) that limit the strength of crystalline alloys.
One modern amorphous metal, known as Vitreloy, has a tensile strength that is almost twice that of high-grade titanium. However, metallic glasses at room temperature are not ductile and tend to fail suddenly when loaded in tension, which limits the material applicability in reliability-critical applications, as the impending failure is not evident. Therefore, there is considerable interest in producing metal matrix composite materials consisting of a metallic glass matrix containing dendritic particles or fibers of a ductile crystalline metal.
Perhaps the most useful property of bulk amorphous alloys is that they are true glasses, which means that they soften and flow upon heating. This allows for easy processing, such as by injection molding, in much the same way as polymers. As a result, amorphous alloys have been commercialized for use in sports equipment, medical devices, and as cases for electronic equipment. Thin films of amorphous metals can be deposited via high velocity oxygen fuel technique as protective coatings.
Amorphous metals (metallic glasses) exhibit unique softening behavior above their glass transition and this softening has been increasingly explored for thermoplastic forming of metallic glasses. It has been shown that metallic glasses can be patterned on extremely small length scales ranging from 10 nm to several millimeters. It has been suggested that this may solve the problems of nanoimprint lithography where expensive nano-molds made of silicon break easily. Nano-molds made from metallic glasses are easy to fabricate and more durable than silicon molds.
Ti40Cu36Pd14Zr10 is believed to be noncarcinogenic, is about three times stronger than titanium, and its elastic modulus nearly matches bones. It has a high wear resistance and does not produce abrasion powder. The alloy does not undergo shrinkage on solidification. A surface structure can be generated that is biologically attachable by surface modification using laser pulses, allowing better joining with bone.








 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope