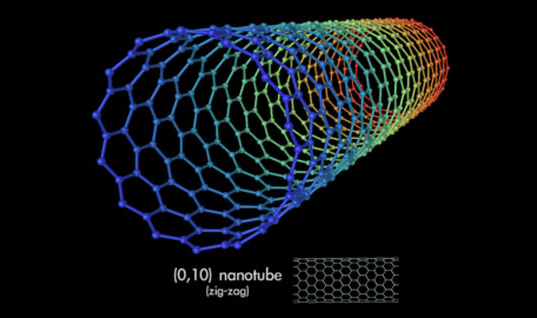
Carbon nanotubes are long chains of carbon held together by the strongest bond in all chemistry, the sacred sp2 bond, even stronger than the sp3 bonds that hold together diamond.
 Carbon nanotubes
Carbon nanotubes
Carbon nanotubes have numerous remarkable physical properties, including ballistic electron transport (making them ideal for electronics) and so much tensile strength that they are the only substance that could be used to build a space elevator.
The specific strength of carbon nanotubes is 48,000 kN·m/kg, the best of known materials, compared to high-carbon steel’s 154 kN·/kg. That’s 300 times stronger than steel. You could build towers hundreds of kilometers high with it.
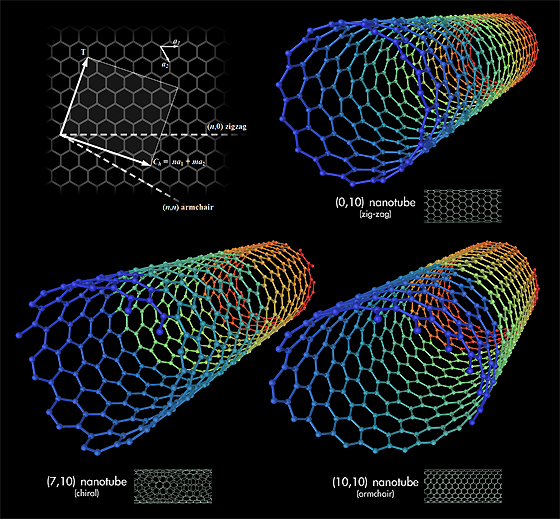 Carbon nanotubes are long chains of carbon held together
Carbon nanotubes are long chains of carbon held together
Creating the perfect solar cell (i.e. a cell that’s both efficient and cheaply produced) is certainly a work in progress. Researchers across the world have attempted to create cells from silicon, plastic and even human hair!
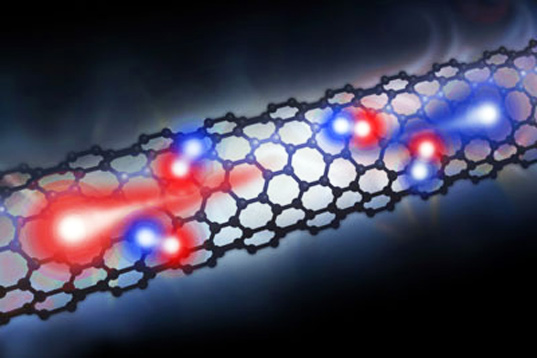 Carbon Nanotubes Could Create Better Solar Cells
Carbon Nanotubes Could Create Better Solar Cells
Now, researchers at Cornell University came up with another concept : crafting solar cells from carbon nanotubes. Though still in the very early stages of development, if perfected, carbon nanotube-based cells could provide a more efficient method of converting light to electricity.
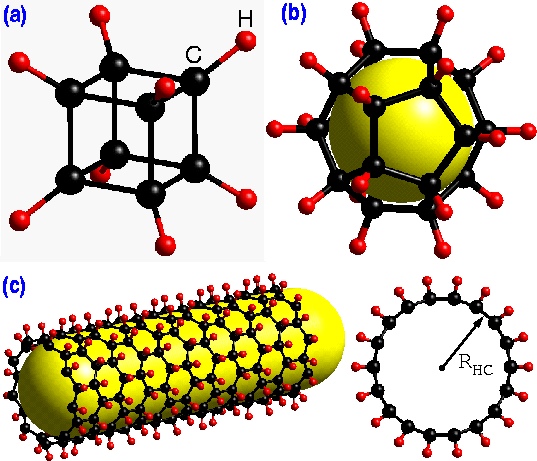
Three different polyhedra of carbon and hydrogen; (a) cubane, (b) dodecahedrane, and (c) a side and top view of a single-wall exohydrogenated carbon nanotube.
Carbon nanotubes exhibit very unusual structural and electronic properties, suggesting a wide variety of technological applications, including the storage of hydrogen where the large effective surface area promises a large absorption capacity. Unfortunately, the studies to date report conflicting results.
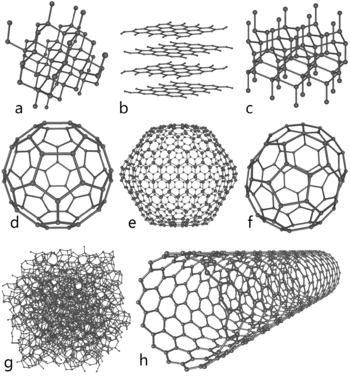 Some allotropes of carbon. Compare the nanotube (h) to: a) diamond; b) graphite; c) lonsdaleite; d-f) fullerenes (C60, C540, C70); g) amorphous carbon.
Some allotropes of carbon. Compare the nanotube (h) to: a) diamond; b) graphite; c) lonsdaleite; d-f) fullerenes (C60, C540, C70); g) amorphous carbon.
While some labs report hydrogen storage densities up to 10 wt\% other labs report only 0.4 wt\% on the same system. Theories based on physisorption have failed to predict such high uptake. To the best of our knowledge, studies of hydrogen chemisorption in nanotubes are very limited and are clearly needed to have a better understanding of hydrogen and nanotube system.
Hydrogen-carbon interactions have been studied extensively both theoretically and experimentally for many interesting polyhedral molecules, such as cubane (C8H8), dodecahedrane (C20H20), and various isomers of C60Hn (see Fig. Above) Despite its very strained 90o CCC-bond angle cubane has been synthesized successfully. Similarly, dodecahedrane and various isomers of C60Hn (up to n=32) have been also synthesized.
These novel polyhedral molecules which represent the zero dimensional case, exhibit many interesting properties. However, due to the one dimensional nature and the curvature of carbon nanotubes, the hydrogen-carbon interactions in these systems may be quite different than those in polyhedral molecules. Therefore, it is important to know if it is also possible to hydrogenate carbon nanotubes in a similar way and if so what their structural and electronic properties would be.
This paper addresses this important issue by performing extensive first-principles calculations and shows that the chemisorption of hydrogen is dependent on the radius and chirality of the nanotubes. Theoretical predictions from first-principles studies played an important role in guiding experimental studies in the past and we expect that many findings reported here may have important implications in this interesting system as well.
You might also like
| What is Damascus Steel ? Damascus steel is one of the greatest wonders... | Timeline of materials technology BC 29,000–25,000 BC – First pottery... | What is Hybrid Materials? Hybrid materials are composites consisting... | Application of Nanotechnology With nanotechnology, a large set of materials... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope