A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous (e.g., a glass). Because most common ceramics are crystalline, the definition of ceramic is often restricted to inorganic crystalline materials, as opposed to the noncrystalline glasses.
 Advanced Ceramic Materials
Advanced Ceramic Materials
The earliest ceramics were pottery objects made from clay, either by itself or mixed with other materials, hardened in fire. Later ceramics were glazed and fired to create a colored, smooth surface. Ceramics now include domestic, industrial and building products and art objects. In the 20th century, new ceramic materials were developed for use in advanced ceramic engineering; for example, in semiconductors.
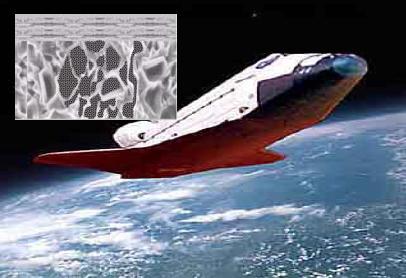 Advance ceramics application
Advance ceramics application
Ceramics, as is pointed out in the article ceramic composition and properties, are traditionally described as inorganic, nonmetallic solids that are prepared from powdered materials, are fabricated into products through the application of heat, and display such characteristic properties as hardness, strength, low electrical conductivity, and brittleness. Advanced ceramics represent an “advancement” over this traditional definition. Through the application of a modern materials science approach, new materials or new combinations of existing materials have been designed that exhibit surprising variations on the properties traditionally ascribed to ceramics. As a result, there are now ceramic products that are as tough and electrically conductive as some metals. Developments in advanced ceramic processing continue at a rapid pace, constituting what can be considered a revolution in the kind of materials and properties obtained.
Technical ceramics can also be classified into three distinct material categories :
- Oxides : alumina, beryllia, ceria, zirconia. Oxidation resistant, chemically inert, electrically insulating, generally low thermal conductivity, slightly complex manufacturing and low cost for alumina, more complex manufacturing and higher cost for zirconia.
- Nonoxides : carbide, boride, nitride, silicide. Low oxidation resistance, extreme hardness, chemically inert, high thermal conductivity, and electrically conducting, difficult energy dependent manufacturing and high cost.
- Composite materials : particulate reinforced, fiber reinforced, combinations of oxides and nonoxides.Toughness, low and high oxidation resistance (type related), variable thermal and electrical conductivity, complex manufacturing processes, high cost.
The 20th century has produced the greatest advancement in ceramics and materials technology since humans have been capable of conceptive thought. The extensive metallurgical developments in this period have now produced almost every conceivable combination of metal alloys and the capabilities of those alloys are fairly well known and exploited. The push for ever faster, more efficient, less costly production techniques continues today. As the limits of metal-based systems are surpassed, new materials capable of operating under higher temperatures, higher speeds, longer life factors and lower maintenance costs are required to maintain pace with technological advancements.
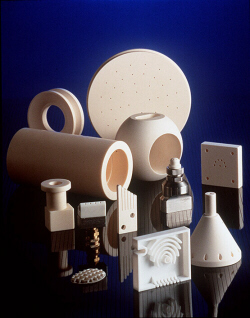 High purity alumina, zirconia and machinable ceramics
High purity alumina, zirconia and machinable ceramics
Metals, by virtue of their unique properties: ductility, tensile strength, abundance, simple chemistry, relatively low cost of production, case of forming, case of joining, etc. have occupied the vanguard position in regard to materials development. By contrast ceramics: brittle by nature, having a more complex chemistry and requiring advanced processing technology and equipment to produce, perform best when combined with other materials, such as metals and polymers which can be used as support structures. This combination enables large shapes to be made; the Space Shuttle is a typical example of the application of advanced materials and an excellent example of the capability of advanced materials.
 Advance Ceramics at Common Rail Fuel Pump with Cam Roller
Advance Ceramics at Common Rail Fuel Pump with Cam Roller
Ceramics for today’s engineering applications can be considered to be non-traditional. Traditional ceramics are the older and more generally known types, such as: porcelain, brick, earthenware, etc. The new and emerging family of ceramics are referred to as advanced, new or fine, and utilise highly refined materials and new forming techniques.
 Advanced Ceramics Cutlery Kitchen Tools
Advanced Ceramics Cutlery Kitchen Tools
These “new” or “advanced” ceramics, when used as an engineering material, posses several properties which can be viewed as superior to metal-based systems. These properties place this new group of ceramics in a most attractive position, not only in the area of performance but also cost effectiveness. These properties include high resistance to abrasion, excellent hot strength, chemical inertness, high machining speeds (as tools) and dimensional stability.
Oxide Ceramics
High purity starting materials (powders) are prepared using mineral processing techniques to produce a concentrate followed by further processing (typically wet chemistry) to remove unwanted impurities and to add other compounds to create the desired starting composition. This is a most important stage in the preparation of high performance oxide ceramics. As these are generally high purity systems minor impurities can have a dynamic effect, for example small amounts of MgO can have a marked effect upon the sintering behaviour of alumina.
Various heat treatment procedures are utilised to create carefully controlled crystal structures. These powders are generally ground to an extremely fine or “ultimate” crystal size to assist ceramic reactivity. Plasticisers and binders are blended with these powders to suit the preferred method of forming (pressing, extrusion, slip casting, etc.) to produce the “raw” material. Both high and low-pressure forming techniques are used. The raw material is formed into the required “green” shape or precursor (machined or turned to shape if required) and fired to high temperatures in air or a slightly reducing atmosphere to produce a dense product.
Non-Oxide Ceramics
The production of non-oxide ceramics is usually a three stage process involving: first the preparation of precursors or starting powders, secondly the mixing of these precursors to create the desired compounds (Ti + 2B, Si + C, etc.) and thirdly the forming and sintering of the final component. The formation of starting materials and firing for this group, require carefully controlled furnace or kiln conditions to ensure the absence of oxygen during heating as these materials will readily oxidise during firing. This group of materials generally requires quite high temperatures to effect sintering. Similar to oxide ceramics, carefully controlled purities and crystalline characteristics are needed to achieve the desired final ceramic properties.
Ceramic-Based Composites
This group can be composed of a combination of: oxide ceramics – non-oxide ceramics (granular, platy, whiskers, etc.), oxide - oxide ceramics, non-oxide – non-oxide ceramics, ceramics - polymers, etc. an almost infinite number of combinations are possible. The object is to improve either the toughness or hardness to be more suited to a particular application. This is a somewhat new area of development and compositions can also include metals in particulate or matrix form.
Advanced ceramic materials are now well established in many areas of every day use. The improvements in performance, service life, savings in operational costs and savings in maintenance are clear evidence of the benefits of advanced ceramic materials. Life expectancies, now in years instead of months with cost economics in the order of only double existing component costs, give advanced ceramics materials a major advantage. The production of these advanced materials is a complex and demanding process with high equipment costs and the requirement of highly specialised and trained people. The ceramic materials of tomorrow will exploit the properties of polycrystalline phase combinations and composite ceramic structures, i.e. the co-precipitation or inclusion of differing crystalline structures having beneficial properties working together in the final compound.
Tomorrow (even today) the quest will be to pack the highest amount of bond energy into the final ceramic compound and to impart a high degree of ductility or elasticity into those bonds. This energy level has to be exceeded to cause failure or dislocation. The changing pace of technology and materials also means that newer compounds precisely engineered to function will be developed. Just how this will be achieved and when the knowledge becomes public - who can tell! Ceramics, an old class of material, still present opportunities for new material developments.
You might also like
| Types of Materials Metals: Metals are elements... | Metallurgy Glossary Metallurgy Glossary Activity: A function... | Metallography This image titled "Wood Pile (SEM)" received... | Metal Spraying Metal spraying is spraying hot metal... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope