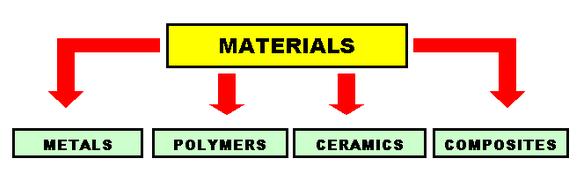
Metals:
Metals are elements that generally have good electrical and thermal conductivity.Many metals have high strength, high stiffness, and have good ductility. Some metals, such as iron, cobalt and nickel are magnetic.At extremely low temperatures, some metals and intermetallic compounds become superconductors.
Pure metals:
Pure metals are elements which comes from a particular area of the periodic table. Examples of pure metals include copper in electrical wires and aluminum in cooking foil and beverage cans.

Metal Alloys:
Metal Alloys contain more than one metallic element. Their properties can be changed by changing the elements present in the alloy. Examples of metal alloys include stainless steel which is an alloy of iron, nickel, and chromium and gold jewelry which usually contains an alloy of gold and nickel.
The most important properties of metals include density, fracture toughness, strength and plastic deformation. The atomic bonding of metals also affects their properties. In metals, the outer valence electrons are shared among all atoms, and are free to travel everywhere. Since electrons conduct heat and electricity, metals make good cooking pans and electrical wires. Many metals and alloys have high densities and are used in applications which require a high mass-to-volume ratio. Some metal alloys, such as those based on Aluminum, have low densities and are used in aerospace applications for fuel economy.Many metal alloys also have high fracture toughness, which means they can withstand impact and are durable.
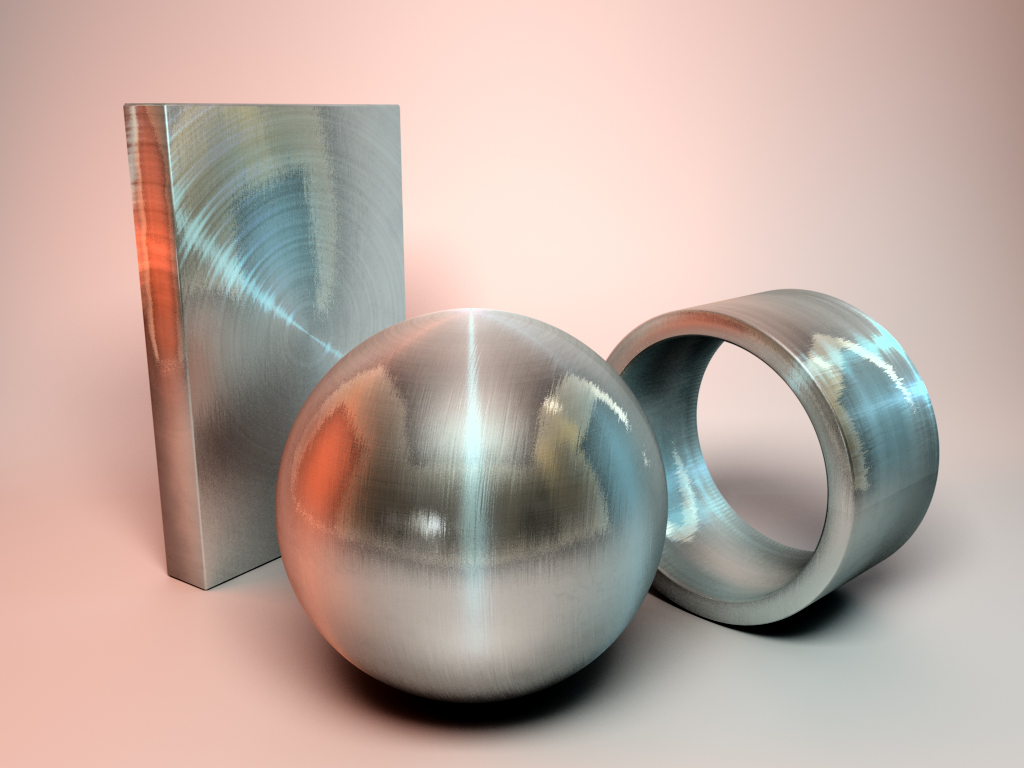
Polymers
A polymer has a repeating structure, usually based on a carbon backbone. The repeating structure results in large chainlike molecules.Polymers are useful because they are lightweight, are corrosion resistant, are easy to process at low temperatures, and are generally inexpensive.
Some important characteristics of polymers include their size (or molecular weight), softening and melting points, crystallinity, and structure. The mechanical properties of polymers generally include low strength and high toughness. Their strength is often improved using reinforced composite structures. One of the distinct properties of polymers is that they are poor conductors of electricity and heat, which makes them good insulators.
Ceramics
Ceramic is often broadly defined as any inorganic nonmetallic material. Examples of such materials can be anything from NaCl (table salt) to clay (a complex silicate). Some of the useful properties of ceramics and glasses include high melting temperature, low density, high strength, stiffness, hardness, wear resistance, and corrosion resistance.
Many ceramics are good electrical and thermal insulators. Some ceramics have special properties: some ceramics are magnetic materials; some are piezoelectric materials; and a few special ceramics are superconductors at very low temperatures. Ceramics and glasses have one major drawback: they are brittle.
Glasses
A glass is an inorganic nonmetallic material that does not have a crystalline structure. Such materials are said to be amorphous. Examples of glasses range from the soda-lime silicate glass in soda bottles to the extremely high purity silica glass in optical fibers.
Picture Credit : Fanpop.com
Composites
Composites are formed from two or more types of materials. Examples include polymer/ceramic and metal/ceramic composites.
Composites are used because overall properties of the composites are superior to those of the individual components. For example: polymer/ceramic composites have a greater modulus than the polymer component, but aren’t as brittle as ceramics.
You might also like
Random Posts
- Characterization of Materials
Characterization, when used in materials science, refers to the use of external techniques to probe into the internal st...
- How Aluminum is Produced
Aluminum manufacture is accomplished in two phases: the Bayer process of refining the bauxite ore to obtain aluminum oxi...
- The Nature Inspired Innovation part 2
If you've ever tried to pick a mussel off a rock or pier piling, you've likely noticed that they sure know how to stick ...
- Extrusion
Extrusion is the process by which long straight metal parts can be produced. The cross-sections that can be produced var...
- Advanced Ceramics
Ceramics, as is pointed out in the article ceramic composition and properties, are traditionally described as inorganic,...
















 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope