What is Cementite ?
Cementite is iron carbide with the formula Fe3C, and an orthorhombic crystal structure. It is a hard, brittle material, essentially a ceramic in its pure form. It forms directly from the melt in the case of white cast iron. In carbon steel, it either forms from austenite during cooling or from martensite during tempering.
Cementite, also known as iron carbide, is a chemical compound of iron and carbon, with the formula Fe3C (or Fe2C:Fe). By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure.
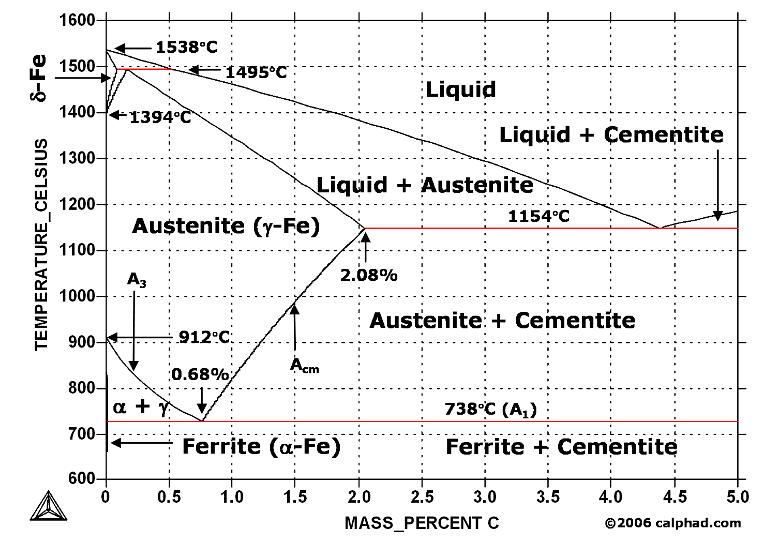
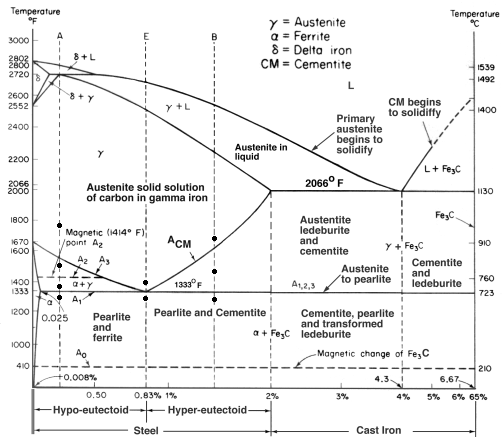
Cementite contains 6.67% Carbon by weight; thus above that carbon content in the Fe-C phase system, the alloy is no longer steel or cast iron, as all of the available iron is contained in cementite. Cementite mixes with ferrite, the other product of austenite, to form lamellar structures called pearlite and bainite. Much larger lamellae, visible to the naked eye, make up the structure of Damascus steel. Fe3C is also known as cohenite, particularly when found mixed with nickel and cobalt carbides in meteorites.
In the iron–carbon system (i.e. plain-carbon steels and cast irons) it is a common constituent because ferrite can contain at most 0.02wt% of uncombined carbon. Therefore, in carbon steels and cast irons that are slowly cooled a portion of the elements is in the form of cementite. It forms directly from the melt in the case of white cast iron. In carbon steel, it either forms from austenite during cooling or from martensite during tempering. An intimate mixture with ferrite, the other product of austenite, forms a lamellar structure called pearlite.
Microstructure of Cementite
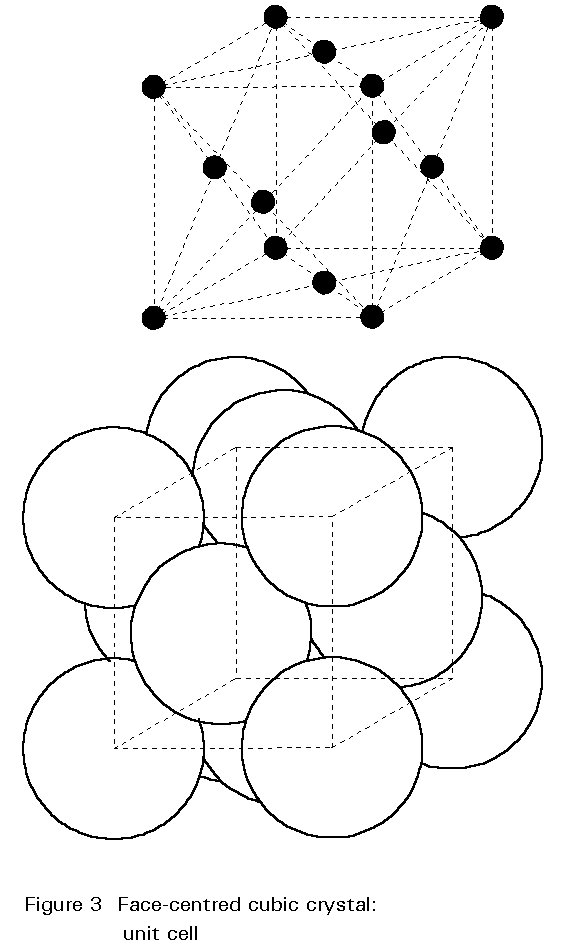
Cementite is a chemical compound whose inclusion hardens steel. Each molecule is made of three iron atoms bonded to one carbon atom (Fe3C) to form a crystal lattice structure called orthorhombic, where multiple rectangular prisms arise from the same base structure and intersect at 90 degree angles. The result is a very hard and brittle substance called iron carbide, or cementite.
In its purest form, cementite is classified as a non-oxide ceramic. It is solid and inert, and can withstand crushing force, chemical erosion, abrasion, and temperatures up to 3000 degrees F (1600 C). It forms naturally by the melting of white cast iron, where it precipitates out of the iron as carbon to form large particles. It sometimes appears this way in phase with austenite, an allotrope of iron, which can sometimes cool to form martensite, a steel with a very strong crystal lattice.
Steel is tempered to increase hardness and reduce brittleness by creating cementite. The first step in the tempering process is called austenizing, when the steel is melted into a solution of iron and carbon, or austenite. The steel is rapidly cooled, and martensite forms from the austenite. It is then heated again, and cooled slowly in a controlled manner, and cementite is formed.





 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope