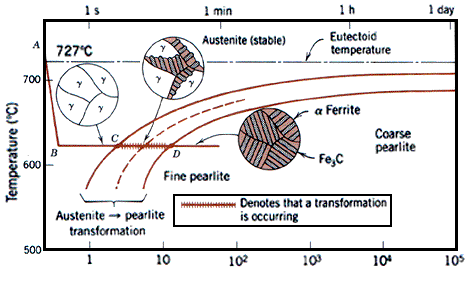
 Fe-Fe3C T-T-T Diagram, Adapted from Callister pg. 295, Fig. 10.6
Fe-Fe3C T-T-T Diagram, Adapted from Callister pg. 295, Fig. 10.6
The time-temperature transformation curves correspond to the start and finish of transformations which extend into the range of temperatures where austenite transforms to pearlite. Above 550 C, austenite transforms completely to pearlite. Below 550 C, both pearlite and bainite are formed and below 450 C, only bainite is formed.
The horizontal line C-D that runs between the two curves marks the beginning and end of isothermal transformations. The dashed line that runs parallel to the solid line curves represents the time to transform half the austenite to pearlite. Below we have listed some simple examples as an exercise at other temperatures that result in different phase transformations and hence different microstructures.
Problem Solving :
Given figure below, describe what transformations happen in :
Given figure below, describe what transformations happen in :
a. Path 1 (Red line) b. Path 2 (Green line) c. Path 3 (Blue line) d. Path 4 (Orange line)
Time-Temperature Paths on Isothermal Transformation Diagram
Solution :
a. (Red) The specimen is cooled rapidly to 433 K and left for 20 minutes. The cooling rate is too rapid for pearlite to form at higher temperatures; therefore, the steel remains in the austenitic phase until the Ms temperature is passed, where martensite begins to form. Since 433 K is the temperature at which half of the austenite transforms to martensite, the direct quench converts 50% of the structure to martensite. Holding at 433 K forms only a small quantity of additional martensite, so the structure can be assumed to be half martensite and half retained austenite.
b. (Green) The specimen is held at 523 K for 100 seconds, which is not long enough to form bainite. Therefore, the second quench from 523 K to room temperature develops a martensitic structure.
c. (Blue) An isothermal hold at 573 K for 500 seconds produces a half-bainite and half-austenite structure. Cooling quickly would result in a final structure of martensite and bainite.
d. (Orange) Austenite converts completely to fine pearlite after eight seconds at 873 K. This phase is stable and will not be changed on holding for 100,000 seconds at 873 K. The final structure, when cooled, is fine pearlite.
You might also like
| Phase Diagram of Steel Fe-Fe3C Phase Diagram, Materials Science... | Time-Temperature-Transformation (TTT ) Diagram T (Time) T(Temperature) T(Transformation)... | Bainite Bainite is an acicular microstructure... | Cast Iron Cast iron is derived from pig iron,... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope
That is a really good read for me, must admit that you’re one of the best bloggers I ever saw. Many thanks for posting this useful piece of content.