Influence of Temperature on Crystal Structure
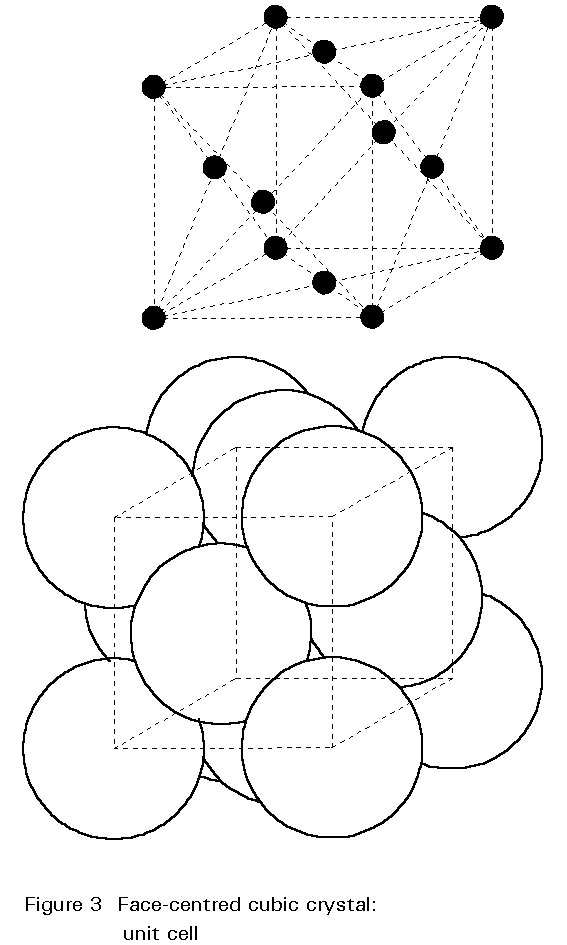
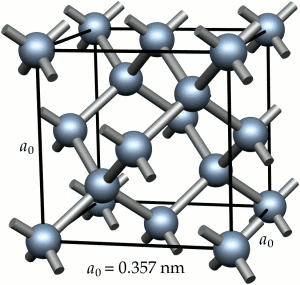
The crystal structure of steel changes with increasing temperature. For pure iron this change occurs at 910° C. The body-centred cubic (bcc) crystals of Figure 2 change to face-centred cubic (fcc) crystals as illustrated in Figure 3. For fcc crystals the atoms of iron are on the cube corners and at the centres of each face of the cube. The body-centred position is empty.
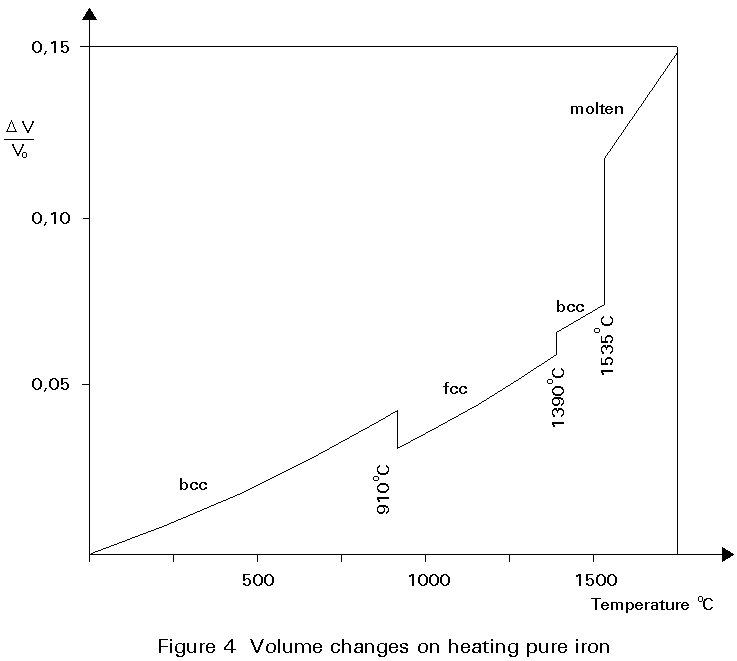
A given number of atoms occupy slightly less volume when arranged as fcc crystals than when arranged as bcc crystals. Thus the change of the crystal structure is accompanied by a volume change. This change is illustrated in Figure 4. When a piece of pure iron is heated, expansion occurs in the normal way until the temperature of 910° C is reached. At this temperature there is a step contraction of about ½% in volume associated with the transformation from the bcc to fcc crystal structure. Further heating gives further thermal expansion until, at about 1400°C the fcc structure reverts to the bcc form and there is a step expansion which restores the volume lost at 910°C. Heating beyond 1400°C gives thermal expansion until melting occurs at 1540°C. The curve is reversible on cooling slowly.
The property that metals may have different crystal structures, depending on temperature, is called allotropy.
Solution of Carbon in bcc and fcc Crystals
When the atoms of two materials A and B have about the same size, crystal structures may be formed where a number of the A atoms are replaced by B atoms. Such a solution is called substitutional because one atom substitutes for the other. An example is nickel in steel.
When the atoms of two materials have a different size, the smaller atom may be able to fit between the bigger atoms. Such a solution is called interstitial. The most familiar example is the solution of carbon in iron. In this way the high temperature fcc crystals can contain up to 2% solid solution carbon at 1130°C, while in the low temperature bcc crystals, the maximum amount of carbon which can be held in solution is 0,02% at 723°C and about 0,002% at ambient temperature.
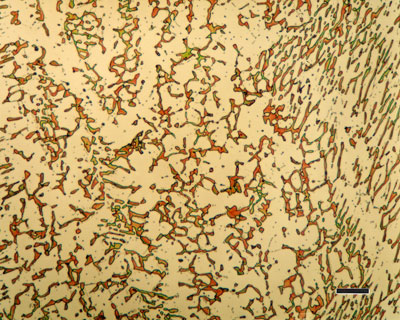
Thus a steel containing 0,5% carbon, for example, can dissolve all the carbon in the higher temperature fcc crystals but on cooling cannot maintain all the carbon in solution in the bcc crystals. The surplus of carbon reacts with iron to form iron carbide (Fe3C), usually called cementite. Cementite is hard and brittle compared to pure iron.
The amount of cementite and the distribution of cementite particles in the microstructure is important for the engineering properties of steel.
Nomenclature
The following nomenclature is used by the metallurgist:
- Ferrite or a -FeThe bcc form of iron in which up to 0,02%C by weight may be dissolved.
- CementiteIron carbide Fe3C (which contains about 6,67%C).
- PearliteThe laminar mixture of ferrite and cementite described earlier. The overall carbon content of the mixture is 0,8% by weight.
- Austenite or g -FeThe fcc form of iron which exists at high temperatures and which can contain up to approximately 2%C by weight.
- SteelAlloys containing less than 2% carbon by weight.
- Cast IronAlloys containing more than 2% carbon by weight.
Steel used in structures such as bridges, buildings and ships, usually contains between 0,1% and 0,25% carbon by weight.
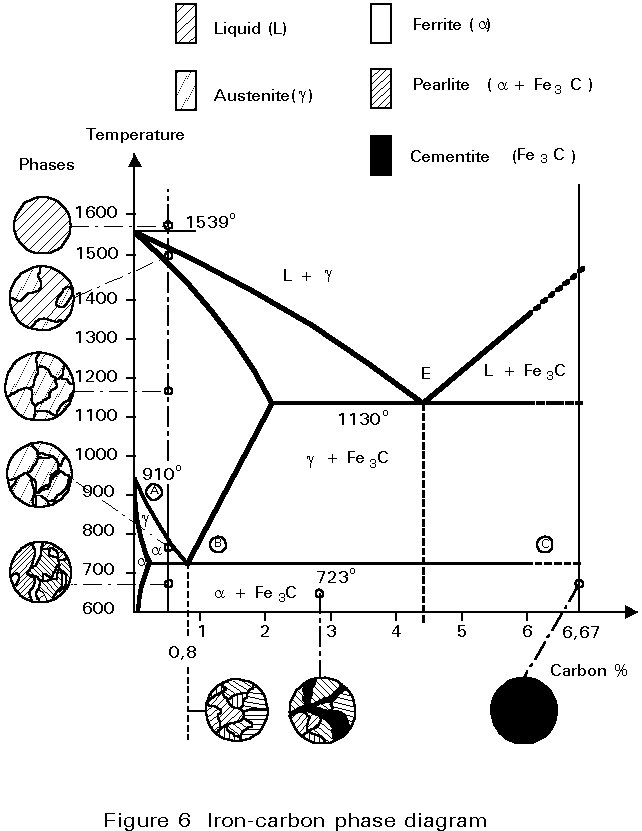
The Iron-Carbon Phase Diagram
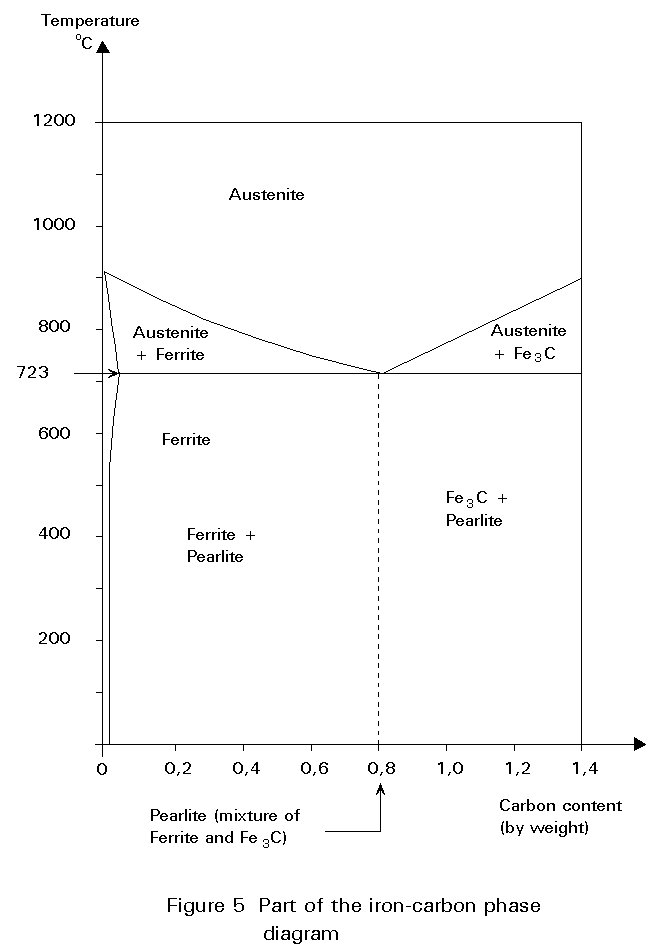
The iron-carbon phase diagram is essentially a map. The most important part is shown in Figure 5. More details are given in Figure 6.
Any point in the field of the diagram represents a steel containing a particular carbon content at a particular temperature. The diagram is divided into areas showing the structures that are stable at particular compositions and temperatures. The diagram may be used to consider what happens when a steel of 0,5%C is cooled from 1000°C (Figure 6). At 1000°C the structure is austenite, i.e. polycrystalline fcc crystals with all the carbon dissolved in them. No change occurs on cooling until the temperature reaches about 800°C. At this temperature, a boundary is crossed from the field labelled Austenite (g) to the field labelled Ferrite + Austenite (a + g), i.e. some crystals of bcc iron, containing very little carbon, begin to form from the fcc iron. Because the ferrite contains so little carbon, the carbon left must concentrate in the residual austenite. The carbon content of the austenite and the relative proportions of ferrite and austenite in the microstructure adjust themselves to maintain the original overall carbon content.
These quantities may be worked out by considering the expanded part of the iron-carbon diagram shown in Figure 7. Imagine that the steel has cooled to 750°C. The combination of overall carbon content and temperature is represented by point X.
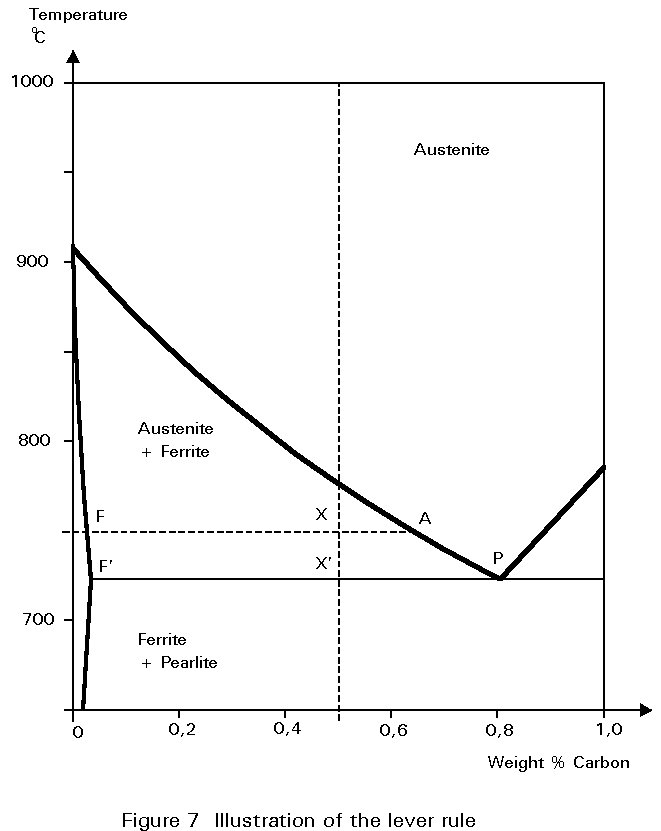 All the constituents of the microstructure are at the same temperature. A line of constant temperature may be drawn through X. It cuts the boundaries of the austenite and ferrite field at F and A. These intercepts give the carbon contents of ferrite and austenite respectively at the particular temperature. If, now, the line FA is envisaged as a rigid beam which can rotate about a fulcrum at X, the ‘weight’ of austenite hanging at A must balance the ‘weight’ of ferrite hanging at F.
All the constituents of the microstructure are at the same temperature. A line of constant temperature may be drawn through X. It cuts the boundaries of the austenite and ferrite field at F and A. These intercepts give the carbon contents of ferrite and austenite respectively at the particular temperature. If, now, the line FA is envisaged as a rigid beam which can rotate about a fulcrum at X, the ‘weight’ of austenite hanging at A must balance the ‘weight’ of ferrite hanging at F.
This is the so-called Lever Rule:
Weight of ferrite ´ FX = Weight of austenite ´ AX
The ratio of ferrite to austenite in the microstructure is then given by:
Thus, as the steel cools, the proportion of ferrite increases and the carbon content of the remaining austenite increases, until cooling reaches 723°C. At this temperature the carbon content of the austenite is 0,8% and it can take no more. Cooling to just below this temperature causes the austenite to decompose. It decomposes into the lamellar mixture of ferrite and Fe3C identified earlier as pearlite.
The proportions of ferrite and pearlite in the microstructure, say at 722°C, are virtually the same as the proportions of ferrite and austenite immediately before the decomposition at 723°C.
Thus, referring to Figure 7 and using the Lever Rule:
Weight of ferrite ´ F¢ X¢ = Weight of pearlite ´ F¢ P
In this case, there should be about twice as much pearlite as ferrite.
For other steels containing less than 0,8%C, the explanation is identical except for the proportions of pearlite in the microstructure below 723°C. This varies approximately linearly with carbon content between zero at 0,02%C and 100% at 0,8%C. A typical mild steel containing 0,2%C would contain about 25% pearlite.
For steels containing a greater percentage of carbon than 0,8%, the structure is fully austenitic on cooling from high temperatures. The first change to occur is the formation of particles of Fe3C from the austenite. This change reduces the carbon content of the residual austenite. On further cooling, the carbon content of the austenite follows the line of the boundary between the g field and g + Fe3C field. Once again, on reaching 723°C the carbon content of the austenite is 0,8%. On cooling further, it decomposes into pearlite as before. Therefore, the final microstructure consists of a few particles of Fe3C embedded in a mass of pearlite.
You might also like
| Metallurgy Glossary Metallurgy Glossary Activity: A function... | Structure and Components of Steel The engineering properties of steel,... | Phase Diagram of Steel Fe-Fe3C Phase Diagram, Materials Science... | Austenite (Gamma Iron) Austenite, also known as gamma phase... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope