Recrystallization Phenomena
What is Recrystallization?
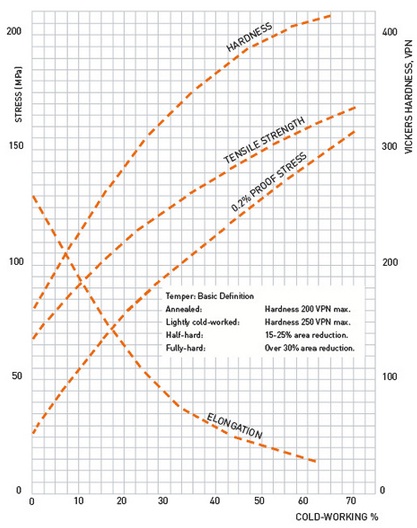
Recrystallization is a process by which deformed grains are replaced by a new set of undeformed grains that nucleate and grow until the original grains have been entirely consumed. Recrystallization is usually accompanied by a reduction in the strength and hardness of a material and a simultaneous increase in the ductility.
Three EBSD maps of the stored energy in an Al-Mg-Mn alloy after exposure to increasing recrystallization temperature. The volume fraction of recrystallized grains (light) increases with temperature for a given time.
Thus, the process may be introduced as a deliberate step in metals processing or may be an undesirable byproduct of another processing step. The most important industrial uses are the softening of metals previously hardened by cold work, which have lost their ductility, and the control of the grain structure in the final product.
Recrystallization may occur during or after deformation (during cooling or a subsequent heat treatment, for example). The former is termed dynamic while the latter is termed static. In addition, recrystallization may occur in a discontinuous manner, where distinct new grains form and grow, or a continuous manner, where the microstructure gradually evolves into a recrystallised microstructure. The different mechanisms by which recrystallization and recovery occur are complex and in many cases remain controversial. The following description is primarily applicable to static continuous recrystallization, which is the most classical variety and probably the most understood. Additional mechanisms include (geometric) dynamic recrystallization and strain induced boundary migration.
Historically it was assumed that the nucleation rate of new recrystallized grains would be determined by the thermal fluctuation model successfully used for solidification and precipitation phenomena. In this theory it is assumed that as a result of the natural movement of atoms (which increases with temperature) small nuclei would spontaneously arise in the matrix. The formation of these nuclei would be associated with an energy requirement due to the formation of a new interface and an energy liberation due to the formation of a new volume of lower energy material. If the nuclei were larger than some critical radius then it would be thermodynamically stable and could start to grow. The main problem with this theory is that the stored energy due to dislocations is very low (0.1-1 Jm?3) while the energy of a grain boundary is quite high (~0.5Jm?2). Calculations based on these values found that the observed nucleation rate was greater than the calculated one by some impossibly large factor (~1050).
Recrystallization of Snow
As a result the alternate theory proposed by Cahn in 1949 is now universally accepted. The recrystallized grains do not nucleate in the classical fashion but rather grow from pre-existing sub-grains and cells. The ‘incubation time’ is then a period of recovery where sub-grains with low-angle boundaries (<1-2°) begin to accumulate dislocations and become increasingly misoriented with respect to their neighbors. The increase in misorientation increases the mobility of the boundary and so the rate of growth of the sub-grain increases. If one sub-grain in a local area happens to have an advantage over its neighbors (such as locally high dislocation densities, a greater size or favorable orientation) then this sub-grain will be able to grow more rapidly than its competitors. As it grows its boundary becomes increasingly misoriented with respect to the surrounding material until it can be recognized as an entirely new strain-free grain. The annealing temperature has a dramatic influence on the rate of recrystallization which is reflected in the above equations. However, for a given temperature there are several additional factors that will influence the rate.
The rate of recrystallization is heavily influenced by the amount of deformation and, to a lesser extent, the manner in which it is applied. Heavily deformed materials will recrystallize more rapidly than those deformed to a lesser extent. Indeed, below a certain deformation recrystallization may never occur. Deformation at higher temperatures will allow concurrent recovery and so such materials will recrystallize more slowly than those deformed at room temperature e.g. contrast hot and cold rolling. In certain cases deformation may be unusually homogeneous or occur only on specific crystallographic planes. The absence of orientation gradients and other heterogeneities may prevent the formation of viable nuclei. Experiments in the 1970s found that molybdenum deformed to a true strain of 0.3, recrystallized most rapidly when tensioned and at decreasing rates for wire drawing, rolling and compression (Barto & Ebert 1971).
The orientation of a grain and how the orientation changes during deformation influence the accumulation of stored energy and hence the rate of recrystallization. The mobility of the grain boundaries is influenced by their orientation and so some crystallographic textures will result in faster growth than others. Solute atoms, both deliberate additions and impurities, have a profound influence on the recrystallization kinetics. Even minor concentrations may have a substantial influence e.g. 0.004% Fe increases the recrystallization temperature by around 100°C (Humphreys and Hatherly 2004). It is currently unknown whether this effect is primarily due to the retardation of nucleation or the reduction in the mobility of grain boundaries i.e. growth.








 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope