What is Anodizing ?
Anodizing is an electrochemical process that converts the metal surface into a decorative, durable, corrosion-resistant, anodic oxide finish. Aluminum is ideally suited to anodizing, although other nonferrous metals, such as magnesium and titanium, also can be anodized.
Anodizing Aluminum
Anodizing, or anodising in British English, is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called “anodizing” because the part to be treated forms the anode electrode of an electrical circuit. Anodizing increases corrosion resistance and wear resistance, and provides better adhesion for paint primers and glues than does bare metal. Anodic films can also be used for a number of cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light.
You might also like
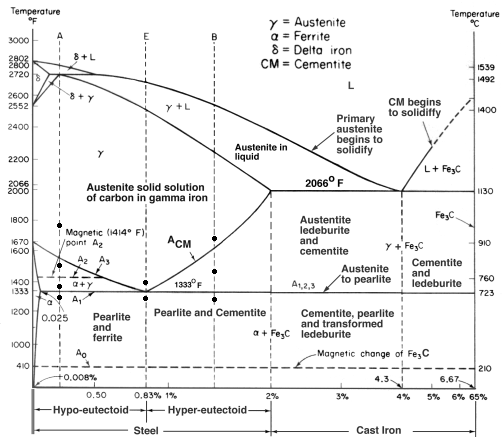
| Corrosion What is Corrosion ? Corrosion is the disintegration... | Iron Carbon Phase Diagram - Fe-Fe3C and T-T-T Diagram Iron Carbon Phase Diagram Iron-carbon... | Gold Plating Gold Electroplating - How it works ? Gold... | Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope