Hydrogen Embrittlement
Hydrogen Embrittlement in carbon steel, zinc plated part. Source:https://www.atclabs.com/
When tensile stresses are applied to a hydrogen embrittled component it may fail prematurely. Hydrogen embrittlement failures are frequently unexpected and sometimes catastrophic. An externally applied load is not required as the tensile stresses may be due to residual stresses in the material. The threshold stresses to cause cracking are commonly below the yield stress of the material. High strength steel, such as quenched and tempered steels or precipitation hardened steels are particularly susceptible to hydrogen embrittlement. Hydrogen can be introduced into the material in service or during materials processing.
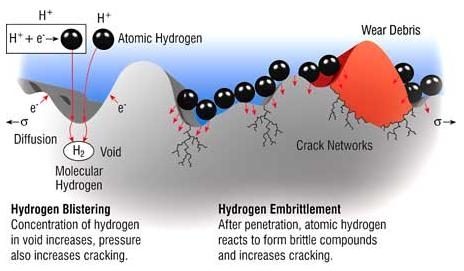 Hydrogen-induced Embrittlement and Blistering Caused by Water Contamination. Source:https://www.machinerylubrication.com
Hydrogen-induced Embrittlement and Blistering Caused by Water Contamination. Source:https://www.machinerylubrication.com
Hydrogen embrittlement is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen. Hydrogen embrittlement is often the result of unintentional introduction of hydrogen into susceptible metals during forming or finishing operations. Hydrogen embrittlement is also used to describe the formation of zircaloy hydride. Use of the term in this context is common in the nuclear industry.
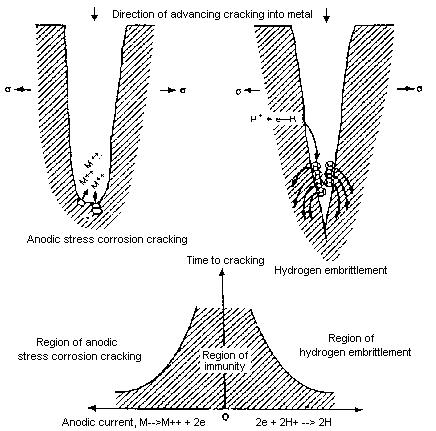 Schematic differentiation of anodic stress corrosion cracking and cathodically sensitive hydrogen embrittlement from Corrosion Source
Schematic differentiation of anodic stress corrosion cracking and cathodically sensitive hydrogen embrittlement from Corrosion Source
The mechanism starts with lone hydrogen atoms diffusing through the metal. At high temperatures, the elevated solubility of hydrogen allows hydrogen to diffuse into the metal (or the hydrogen can diffuse in at a low temperature, assisted by a concentration gradient). When these hydrogen atoms re-combine in minuscule voids of the metal matrix to form hydrogen molecules, they create pressure from inside the cavity they are in. This pressure can increase to levels where the metal has reduced ductility and tensile strength up to the point where it cracks open (hydrogen induced cracking, or HIC). High-strength and low-alloy steels, nickel and titanium alloys are most susceptible.
 Hydrogen Embrittlement of Stainless Steels. Source:https://www.corrosionist.com/
Hydrogen Embrittlement of Stainless Steels. Source:https://www.corrosionist.com/
Tensile stresses, susceptible material, and the presence of hydrogen are necessary to cause hydrogen embrittlement. Residual stresses or externally applied loads resulting in stresses significantly below yield stresses can cause cracking. Thus, catastrophic failure can occur without significant deformation or obvious deterioration of the component.
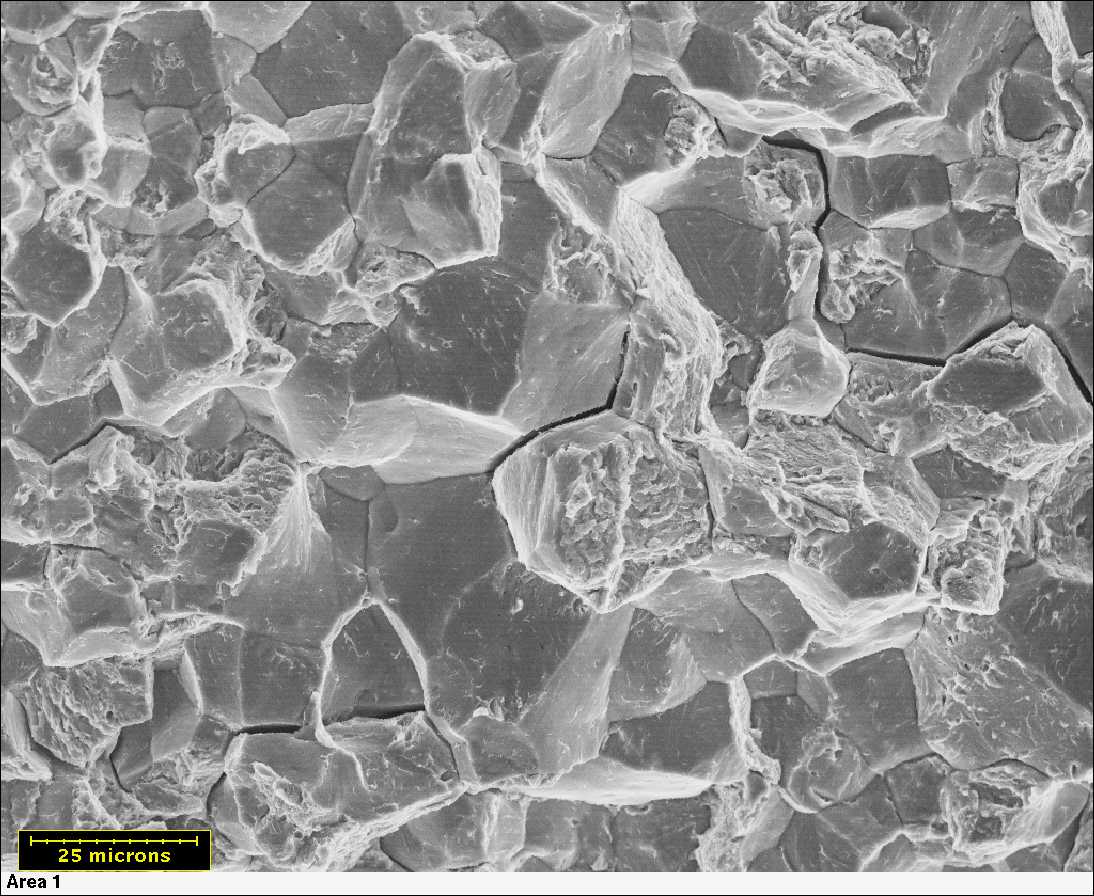
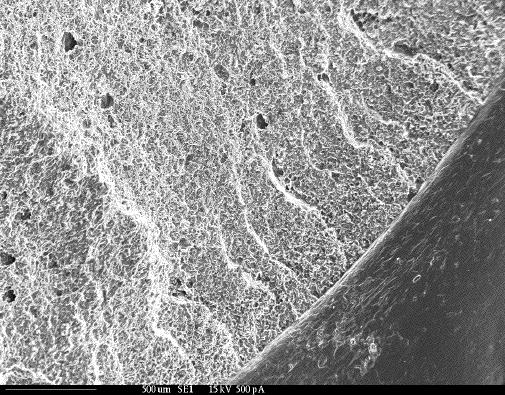 The SEM image is taken from a cellulose-acetate replica made from a fractured steel shaft. The fracture face resembles tension overload of a hydrogen embrittled, tempered, martensitic steel. Source:https://www.corrosionhelp.com/
The SEM image is taken from a cellulose-acetate replica made from a fractured steel shaft. The fracture face resembles tension overload of a hydrogen embrittled, tempered, martensitic steel. Source:https://www.corrosionhelp.com/
Very small amounts of hydrogen can cause hydrogen embrittlement in high strength steels. Common causes of hydrogen embrittlement are pickling, electroplating and welding, however hydrogen embrittlement is not limited to these processes.
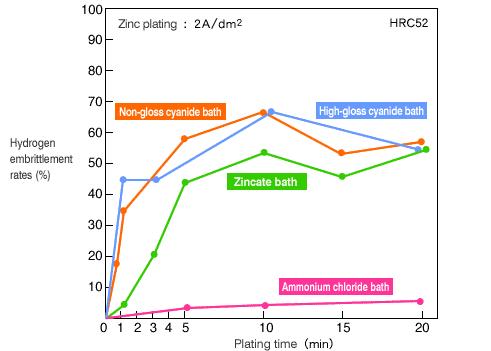 Hydrogen embrittlement rates of zinc plating. Source:https://www.misumi-techcentral.com/
Hydrogen embrittlement rates of zinc plating. Source:https://www.misumi-techcentral.com/
Hydrogen embrittlement is an insidious type of failure as it can occur without an externally applied load or at loads significantly below yield stress. While high strength steels are the most common case of hydrogen embrittlement all materials are susceptible.
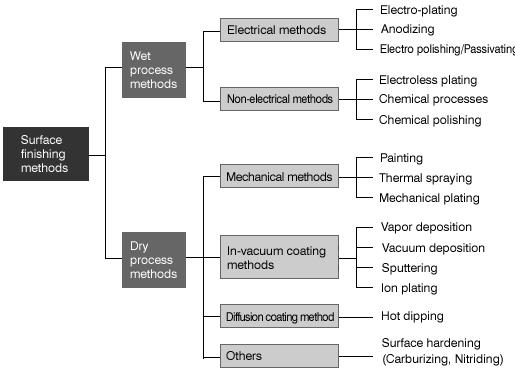 Type of surface finishing processes cause hydrogen embrittlement,source:https://www.misumi-techcentral.com/
Type of surface finishing processes cause hydrogen embrittlement,source:https://www.misumi-techcentral.com/
Austempered iron is also susceptible.Steel with an ultimate tensile strength of less than 1000 MPa or hardness of less than 30 HRC are not generally considered susceptible to hydrogen embrittlement. Jewett et al reports the results of tensile tests carried out on several structural metals under high-pressure molecular hydrogen environment.
 Cyclic Fatigue Failure Caused by Hydrogen Embrittlement,source:https://www.musiccityrodshop.com/
Cyclic Fatigue Failure Caused by Hydrogen Embrittlement,source:https://www.musiccityrodshop.com/
These tests have shown that austenitic stainless steels, aluminum (including alloys), copper (including alloys, e.g. beryllium copper) are not susceptible to hydrogen embrittlement along with few other metals. For example of a severe embrittlement measured by Jewett, the elongation at failure of 17-4PH precipitation hardened stainless steel was measured to drop from 17% to only 1.7% when smooth specimens were exposed to high-pressure hydrogen.
Hydrogen Embrittlement of Valve Capscrew Fasteners - Source:The Hendrix Group (https://www.hghouston.com)
Hydrogen embrittlement can occur during various manufacturing operations or operational use - anywhere that the metal comes into contact with atomic or molecular hydrogen. Processes that can lead to this include cathodic protection, phosphating, pickling, and electroplating.
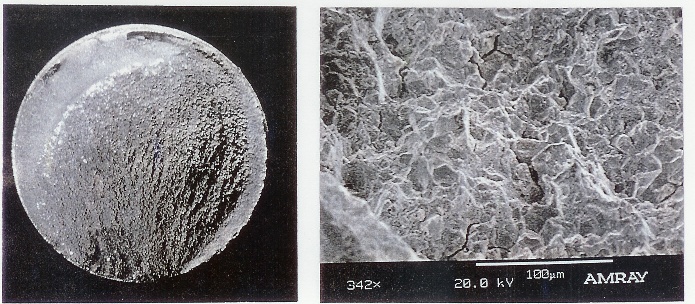
Bolt Failure - Hydrogen Embrittlement. Source:https://www.powerfect.com/
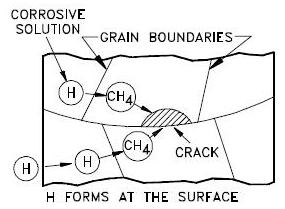
A special case is arc welding, in which the hydrogen is released from moisture (for example in the coating of the welding electrodes; to minimize this, special low-hydrogen electrodes are used for welding high-strength steels). Other mechanisms of introduction of hydrogen into metal are galvanic corrosion, chemical reactions of metal with acids, or with other chemicals (notably hydrogen sulfide in sulfide stress cracking, or SSC, a process of importance for the oil and gas industries).










 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope