Nanotechnology and Cancer
The use of nanotechnology in cancer treatment offers some exciting possibilities, including the possibility of destroying cancer tumors with minimal damage to healthy tissue and organs, as well as the detection and elimination of cancer cells before they form tumors.
 Gold Nanoparticle Kill Cancer
Gold Nanoparticle Kill Cancer
Nanotechnology in general is the study of creating machines under the size of 100 nanometers and the idea, created by Richard P. Feynman in 1959 has progressed from condensing an entire encyclopedia onto the head of a pin into something truly life changing. In more recent times, many impressive new advances are being made in nanotechnology in the field of medicine, allowing doctors to diagnose cancer earlier through advanced imaging and being able to more effectively treat it with more effectively and safer.
Scientists at the Massachusetts Institute of Technology (MIT) in Boston have developed a method of delivering chemotherapy to the cancer cells, while leaving healthy cells undamaged. They dub the device the “smart bomb”. The research is published in this month’s journal Nature.
The scientists use nanotechnology to protect a medicinal supply of chemotherapy and angiogenesis agents until the nanocell enters the tumor. The nanocell is described as a balloon inside a balloon with angiogenesis agents in the outer balloon and chemotherapy in the inner balloon. The angiogenesis agents cause tumors to collapse.
The nanocell’s surface is designed to avoid drawing the attention of the immune system. The nanocells are small enough (only 200 nanometers) to pass through the tumor blood vessels. This therapy has many benefits over standard chemo and radiation therapy. Patients are less likely to suffer nausea, vomiting, hair loss and other side effects. Also, when tested on mice, the mice using this therapy lived longer than their counterparts on traditional chemo and radiation therapies.
Back in September 2004, the US National Cancer Institute (NCI) launched the Alliance for Nanotechnology in Cancer to stimulate and coordinate research in biology, engineering and materials science to push cancer nanotechnology forward. Just over 2 years on, such research is attracting increasing attention: in a round-up of last year’s breakthroughs in the burgeoning field of nanotechnology from Forbes magazine, anticancer nanoparticles featured in the top five.
Most efforts to improve cancer treatment through nanotechnology are at the research or development stage. For example, The Alliance for Nanotechnology in Cancer, established by the U.S. National Cancer Institute, is fostering innovation and collaboration among researchers to resolve some of the major challenges in the application of nanotechnology to cancer.
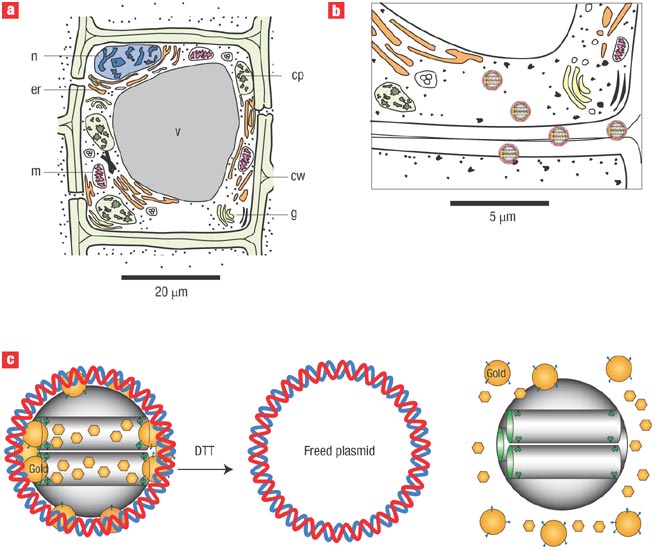
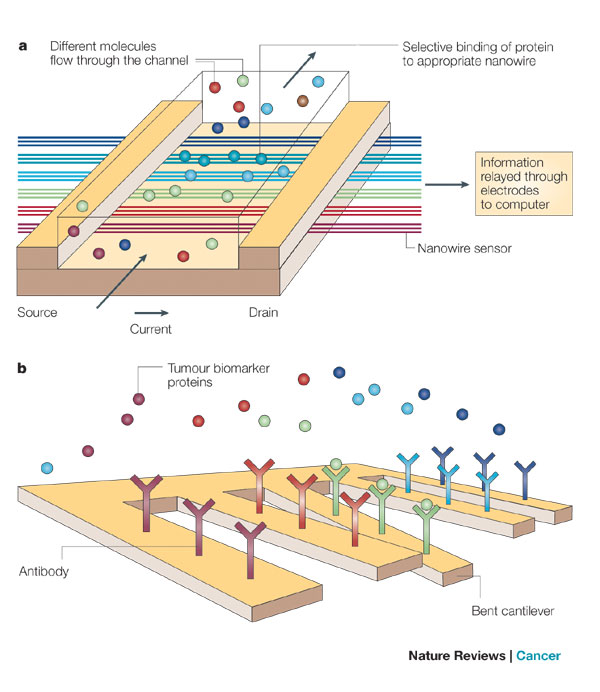 a) Nanowires deployed within a microfluidic system. Different colours indicate that different molecules (coloured circles) adsorb or affinity-bind to different nanowire sensors. The binding causes a change in conductance of the wires, which can be electronically and quantitatively detected in real time. The working principle is that of a (biologically gated) transistor and is illustrated in the insert. The charges of the binding protein disrupt electrical conduction in the underlying nanowire. The ‘nano’ size of the wire is required to attain high signal-to-noise ratios. b) Nanocantilever array. The biomarker proteins are affinity-bound to the cantilevers and cause them to deflect. The deflections can be directly observed with lasers. Alternatively, the shift in resonant frequencies caused by the binding can be electronically detected. As for nanowire sensors, the breakthrough potential in nanocantilever technology is the ability to sense a large number of different proteins at the same time, in real time.
a) Nanowires deployed within a microfluidic system. Different colours indicate that different molecules (coloured circles) adsorb or affinity-bind to different nanowire sensors. The binding causes a change in conductance of the wires, which can be electronically and quantitatively detected in real time. The working principle is that of a (biologically gated) transistor and is illustrated in the insert. The charges of the binding protein disrupt electrical conduction in the underlying nanowire. The ‘nano’ size of the wire is required to attain high signal-to-noise ratios. b) Nanocantilever array. The biomarker proteins are affinity-bound to the cantilevers and cause them to deflect. The deflections can be directly observed with lasers. Alternatively, the shift in resonant frequencies caused by the binding can be electronically detected. As for nanowire sensors, the breakthrough potential in nanocantilever technology is the ability to sense a large number of different proteins at the same time, in real time.
Nanotechnology is being applied to cancer in two broad areas : the development of nanovectors, such as nanoparticles, which can be loaded with drugs or imaging agents and then targeted to tumours, and high-throughput nanosensor devices for detecting the biological signatures of cancer. Spearheading efforts to expedite the clinical application of these technologies, the NCI currently funds eight Centers of Cancer Nanotechnology Excellence (CCNE) in the United States, in addition to 12 other smaller programmes.
It is known that the ability to detect cancerous cells or cells in the precancerous stage relies on the ability to monitor slight changes in molecular composition of the affected cells. Nanotechnology doesn’t just stop at detection but is being developed to assist in the treatment of cancer as well.
Nanotechnology’s versatility is what is key in the treatment of cancer. These devices can be put into the body loaded with targeting information and powerful cancer treating drugs.
The targeting information enables these devices to find the specific cells infected followed by that area being doused with drugs in hope of killing off the cancerous or pre-cancerous cells.
Another hope is giving these devices the ability to release their treatment at specific times (possibly sequentially or simultaneously at different locations) to be even more efficient in cancer treatment.
When nanoshells are in specific cancerous cells, the addition of infrared light creates a temperature increase which is deadly to these cells without causing harm to surrounding cells that do not contain these nanoshells. This has been successful due to the large surface area, enabling researchers to attach lethal amounts of cancer fighting agents while still being small enough to infiltrate cell structures. A single device that is able to find, identify, track and eliminate cancerous and precancerous cells in the body. With advances in nanotechnology occurring daily, this technology is truly advancing to new heights.
Langer, with Omid Farokhzad, Assistant Professor of Anaesthesia at Brigham and Women’s Hospital, Boston, USA, and colleagues, uses polymeric nanoparticles coated with aptamers — RNA-based targeting moieties — to guide them towards the tumour, where they bind, enter the cells and then dissolve to spill out their contents — the anticancer drug docetaxel.
“The size, shape, physical properties, density and charge all affect how particles travel through the body, and whether or not they will cross biological membranes,” says Mauro Ferrari, a professor of nanotechnology at the University of Texas Health Science Center, the M.D. Anderson Cancer Center, and Rice University in Houston, USA. DeSimone has adapted fabrication technologies from the electronics industry to produce shape-specific organic nanoparticles.
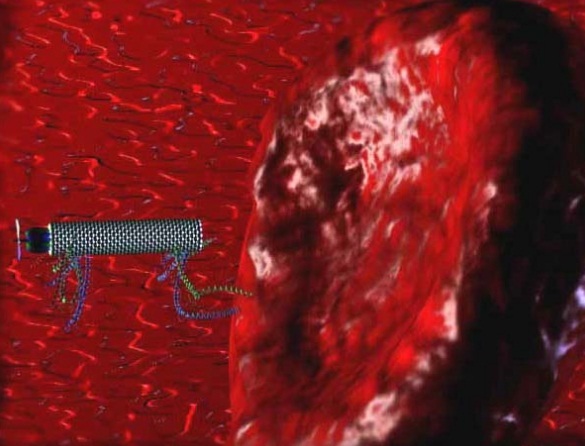
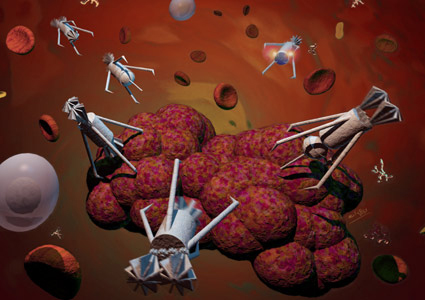 “Drillers, Peepers, Stingers” engage in a delicate surgical operation to remove a cancer tumor. Whilst the Stingers inject a toxin, Drillers cut deep into the tumor. A Peeper broadcasts the whole video scene to the surgeon.
“Drillers, Peepers, Stingers” engage in a delicate surgical operation to remove a cancer tumor. Whilst the Stingers inject a toxin, Drillers cut deep into the tumor. A Peeper broadcasts the whole video scene to the surgeon.
DeSimone’s engineered process allows the precise control over particle size (20 nm to >100 m), particle shape (spheres, cylinders, discs, toroidal), particle composition (organic or inorganic, solid or porous), particle cargo (hydrophilic or hydrophobic therapeutics, biologicals, imaging agents), particle compliance (stiff, deformable) and particle surface properties (Avidin–biotin complexes, targeting peptides, antibodies, aptamers, PEG chains).
Nanoparticles are not the only way to encapsulate a drug or imaging agent into a small carrier, but DeSimone suggests that the nanotechnology approach offers crucial advantages.
DeSimone is also taking a cue from naturally occurring particles, such as red blood cells, to produce compliant nanoparticles that can deform to pass across biological barriers such as sinusoids in the spleen or the blood–brain barrier.
The new Gordon Research Conference on “Cancer Nanotechnology” will allow for further development of a multi-disciplinary research community representing a broad range of disciplines including biology, chemistry, physics, engineering, medicine, and regulatory affairs and supporting the new field of cancer nanotechnology. The ultimate goal of the conference will be to accelerate implementation of nanotechnology innovations into cancer research and its clinical practice.
Cancer is a complex disease. In recent years, nanotechnology has emerged as a “disruptive technology” with a potential to generate new diagnostic and therapeutic products and as a result improve cancer outcomes. Novel and multi-functional nanodevices could be capable of detecting cancer at its earliest stages, delivering anticancer drugs specifically to malignant cells, and determining if these drugs are effective.
Functionalized nanoparticles could deliver multiple therapeutic agents to tumor sites in order to simultaneously attack multiple points in the pathways involved in cancer. The field of cancer nanotechnology is unique. It combines disparate research communities of physical scientists, engineers, and technologists working at the nanoscale with cancer biologists and oncologists specializing in the diagnosis, prevention, and treatment of cancer.
It is common that implementation of new technology discoveries into medical world occurs only when these technologies mature and final medical product is developed. For example, the National Cancer Institute (NCI) has founded the NCI Alliance for Nanotechnology in Cancer - the network of large academic centers performing basic and translational cancer research, Massachusetts Institute of Technology (MIT) is in the process of constructing David Koch Institute for Integrative Cancer Research, Institute of Bioengineering and Nanotechnology has been established in Singapore.
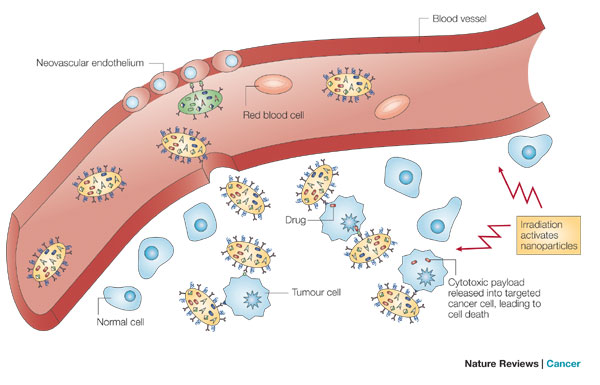 Nanoparticles extravasate into the tumour stroma through the fenestrations of the angiogenic vasculature, demonstrating targeting by enhanced permeation and retention. The particles carry multiple antibodies, which further target them to epitopes on cancer cells, and direct antitumour action. Nanoparticles are activated and release their cytotoxic action when irradiated by external energy. Not shown: nanoparticles might preferentially adhere to cancer neovasculature and cause it to collapse, providing anti-angiogenic therapy. The red blood cells are not shown to scale; the volume occupied by a red blood cell would suffice to host 1–10 million nanoparticles of 10 nm diameter.
Nanoparticles extravasate into the tumour stroma through the fenestrations of the angiogenic vasculature, demonstrating targeting by enhanced permeation and retention. The particles carry multiple antibodies, which further target them to epitopes on cancer cells, and direct antitumour action. Nanoparticles are activated and release their cytotoxic action when irradiated by external energy. Not shown: nanoparticles might preferentially adhere to cancer neovasculature and cause it to collapse, providing anti-angiogenic therapy. The red blood cells are not shown to scale; the volume occupied by a red blood cell would suffice to host 1–10 million nanoparticles of 10 nm diameter.
Liquidia Technologies Inc., based in Morrisville, North Carolina, USA, was spun-off from DeSimone’s laboratory a few years ago to develop this technology platform. Right now, the company is working on feasibility studies, as well as research collaborations with some large pharmaceutical and medical device companies. The synergy between the therapeutic and diagnostic/monitoring applications of nanotechnology could be particularly potent.
You might also like
| What is Nanotechnology? Nanotechnology is the engineering... | What is Nanobots ? How Nanorobots Will Work ? Nanorobotics... | Nanomedicine - the Future Medicine Nanomedicine - Application Nanotechnology Nanomedicine... | What is Nanobiotechnology ? Nanobiotechnology - Meaning and Definition Bionanotechnology... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope