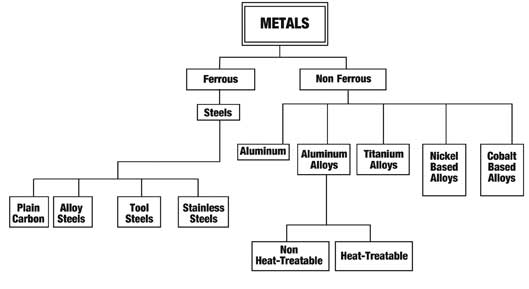
Types of Heat Treatments
Heat Treatment is the controlled heating and cooling of metals to alter their physical and mechanical properties without changing the product shape. Heat treatment is sometimes done inadvertently due to manufacturing processes that either heat or cool the metal such as welding or forming.
 Heat Treatment Processing
Heat Treatment Processing
Heat treating is a group of industrial and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material.
Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering and quenching. It is noteworthy that while the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.
Heat Treatment is often associated with increasing the strength of material, but it can also be used to alter certain manufacturability objectives such as improve machining, improve formability, restore ductility after a cold working operation. Thus it is a very enabling manufacturing process that can not only help other manufacturing process, but can also improve product performance by increasing strength or other desirable characteristics.
Four basic types of heat treatment are used today. They are annealing, normalizing, hardening, and tempering. The techniques used in each process and how they relate to steelworkers are given in the following paragraphs.
HARDENING
Hardening of steels is done to increase the strength and wear properties. If there is sufficient Carbon content then the steel can be directly hardened. The hardening treatment for most steels consists of heating the steel to a set temperature and then cooling it rapidly by plunging it into oil, water, or brine. Most steels require rapid cooling (quenching) for hardening but a few can be air-cooled with the same results. Hardening increases the hardness and strength of the steel, but makes it less ductile. To remove some of the brittleness, you should temper the steel after hardening.
Many nonferrous metals can be hardened and their strength increased by controlled heating and rapid cooling. Pure iron, wrought iron, and extremely low-carbon steels have very little hardening properties and are dif-ficult to harden by heat treatment. Cast iron has limited capabilities for hardening. When you cool cast iron rapidly, it forms white iron, which is hard and brittle. In plain carbon steel, the maximum hardness ob-tained by heat treatment depends almost entirely on the carbon content of the steel.
As the carbon content in-creases, the hardening ability of the steel increases; however, this capability of hardening with an increase in carbon content continues only to a certain point. When you increase the carbon content beyond 0.80 percent, there is no increase in hardness, but there is an increase in wear resistance. When you alloy steel to increase its hardness, the alloys make the carbon more effective in increasing hardness and strength. Because of this, the carbon content required to produce maximum hardness is lower than it is for plain carbon steels. Usually, alloy steels are superior to carbon steels.
Carbon steels are usually quenched in brine or water, and alloy steels are generally quenched in oil. When hardening carbon steel, remember that you must cool the steel to below 1000°F in less than 1 second. Quenching produces extremely high internal stresses in steel, and to relieve them, you can temper the steel just before it becomes cold.
Case Hardening
Case hardening is a thermochemical diffusion process in which an alloying element, most commonly carbon or nitrogen, diffuses into the surface of a monolithic metal. The resulting interstitial solid solution is harder than the base material, which improves wear resistance without sacrificing toughness. Laser surface engineering is a surface treatment with high versatility, selectivity and novel properties. Since the cooling rate is very high in laser treatment, metastable even metallic glass can be obtained by this method.
Case hardening is ideal for parts that require a wear-resistant surface and must be tough enough internally to withstand heavy loading. The steels best suited for case hardening are the low-carbon and low-alloy series. When high-carbon steels are case-hardened, the hardness penetrates the core and causes brittleness. In case hardening, you change the surface of the metal chemically by introducing a high carbide or nitride content. The core remains chemically unaffected. When heat-treated, the high-carbon surface responds to hard-ening, and the core toughens.
Carburizing
Carburizing is a case-harden-ing process by which carbon is added to the surface of low-carbon steel. This results in a carburized steel that has a high-carbon surface and a low-carbon interior. When the carburized steel is heat-treated, the case be-comes hardened and the core remains soft and tough.
Two methods are used for carburizing steel. One method consists of heating the steel in a furnace con-taining a carbon monoxide atmosphere. The other method has the steel placed in a container packed with charcoal or some other carbon-rich material and then heated in a furnace. To cool the parts, you can leave the container in the furnace to cool or remove it and let it air cool. In both cases, the parts become annealed during the slow cooling. The depth of the carbon penetration depends on the length of the soaking period. With to-day’s methods, carburizing is almost exclusively done by gas atmospheres.
Cyaniding
This process is a type of case hardening that is fast and efficient. Preheated steel is dipped into a heated cyanide bath and allowed to soak. Upon removal, it is quenched and then rinsed to remove any residual cyanide. This process produces a thin, hard shell that is harder than the one produced by carburizing and can be completed in 20 to 30 minutes vice several hours. The major drawback is that cyanide salts are a deadly poison.
Nitriding
This case-hardening method pro-duces the hardest surface of any of the hardening processes. It differs from the other methods in that the individual parts have been heat-treated and tempered before nitriding. The parts are then heated in a furnace that has an ammonia gas atmosphere. No quenching is required so there is no worry about warping or other types of distortion. This process is used to case harden items, such as gears, cylinder sleeves, camshafts and other engine parts, that need to be wear resistant and operate in high-heat areas.
Flame Hardening
Flame hardening is another procedure that is used to harden the surface of metal parts. When you use an oxyacetylene flame, a thin layer at the surface of the part is rapidly heated to its critical temperature and then immediately quenched by a combination of a water spray and the cold base metal. This process produces a thin, hardened surface, and at the same time, the internal parts retain their original properties. Whether the process is manual or mechanical, a close watch must be maintained, since the torches heat the metal rapidly and the temperatures are usually determined visually.
TEMPERING
The term tempering is used to describe heating after previously hardening, cold working (cold levelling) or welding to a temperature between room temperature and below the transformation point Ac1 and holding at this temperature with subsequent cooling as suits the purpose (DIN 17022 part 1-5). A microstructure which has been transformed quickly through rapid cooling is not in a stable state of equilibrium meaning that when reheated, the toughness increases and, at the same time, the hardness can be decreased. The amount by which the hardness decreases is determined by the temperature and time period for tempering.
After the hardening treatment is applied, steel is often harder than needed and is too brittle for most practical uses. Also, severe internal stresses are set up during the rapid cooling from the hardening temperature. To relieve the internal stresses and reduce brittle-ness, you should temper the steel after it is hardened. Tempering consists of heating the steel to a specific temperature (below its hardening temperature), holding it at that temperature for the required length of time, and then cooling it, usually instill air. The resultant strength, hardness, and ductility depend on the temperature to which the steel is heated during the tempering process.
To relieve the internal stresses and reduce brittle-ness, you should temper the steel after it is hardened. Tempering consists of heating the steel to a specific temperature (below its hardening temperature), holding it at that temperature for the required length of time, and then cooling it, usually instill air. The resultant strength, hardness, and ductility depend on the temperature to which the steel is heated during the tempering process.
Tempering always follows, never precedes, the hardening operation. Besides reducing brittleness, tempering softens the steel. That is unavoidable, and the amount of hardness that is lost depends on the temperature that the steel is heated to during the tempering process. That is true of all steels except high-speed steel. Tempering increases the hardness of high-speed steel.
Most applications require that quenched parts be tempered. Tempering consists of heating a steel below the lower critical temperature, (often from 400 to 1105 ?F or 205 to 595 ?C, depending on the desired results), to impart some toughness. Tempering may also be performed on normalized steels. Tempering is always conducted at temperatures be-low the low-critical point of the steel. When hardened steel is reheated, temper-ing begins at 212°F and continues as the temperature increases toward the low-critical point. By selecting a definite tempering temperature, you can predetermine the resulting hardness and strength.
The minimum temperature time for tempering should be 1 hour. Normally, the rate of cooling from the tempering temperature has no effect on the steel. Steel parts are usually cooled in still air after being removed from the tempering furnace; however, there are a few types of steel that must be quenched from the tempering tem-perature to prevent brittleness. These blue brittle steels can become brittle if heated in certain temperature ranges and allowed to cool slowly. Some of the nickel chromium steels are subject to this temper brittleness.
Steel that has been freshly ground or polished will form oxide layers when heated. This causes colors to appear on the surface of the steel. As temperature is increased, the iron oxide layer grows in thickness, changing the color. These colors, called tempering colors, have been used for centuries to gauge the temperature of the metal. The tempering colors can be used to judge the final properties of the tempered steel. Very hard tool steel is often tempered in the light to dark straw range, whereas spring steel is often tempered to the blue.
However, the final hardness of the tempered steel will vary, depending on the composition of the steel. Steel may be tempered after being normalized, pro-viding there is any hardness to temper. Annealed steel is impossible to temper. Tempering relieves quenching stresses and reduces hardness and brittleness. Actually, the tensile strength of a hardened steel may increase as the steel is tempered up to a temperature of about 450°F. Tempering increases softness, ductility, malleability, and impact resistance. High-speed steel increases in hardness on temper-ing, provided it is tempered at a high temperature (about 1550°F). Remember, all steel should be removed from the quenching bath and tempered before it is complete] y cold. Failure to temper correctly results in a quick failure of the hardened part.
ANNEALING
Process Annealing is used to treat work-hardened parts made out of low-Carbon steels (< 0.25% Carbon). This allows the parts to be soft enough to undergo further cold working without fracturing. Process annealing is done by raising the temperature to just below the Ferrite-Austenite region, line A1on the diagram. This temperature is about 727 ºC (1341 ºF) so heating it to about 700 ºC (1292 ºF) should suffice. This is held long enough to allow recrystallization of the ferrite phase, and then cooled in still air. Since the material stays in the same phase through out the process, the only change that occurs is the size, shape and distribution of the grain structure. This process is cheaper than either full annealing or normalizing since the material is not heated to a very high temperature or cooled in a furnace.
In general, annealing is the opposite of hardening, You anneal metals to relieve internal stresses, soften them, make them more ductile, and refine their grain structures. Annealing consists of heating a metal to a specific temperature, holding it at that temperature for a set length of time, and then cooling the metal to room temperature. The cooling method depends on the metal and the properties desired. Some metals are furnace-cooled, and others are cooled by burying them in ashes, lime, or other insulating materials.
Soft annealing
Soft annealing is carried out at a temperature of just under Ac1*, sometimes also over Ac1 or by fluctuating around Ac1 with subsequent slow cooling to achieve a soft condition (DIN 17022 part 1-5). Through this heat treatment, the cementite lamination of the perlite is transformed to a spherical form - known as granular cementite. This type of microstructure provides the best workability for steels with a C-content of more than approx. 0.5%. Granular cementite provides the condition for best workability for any type of cold working e.g. for cold-heading, drawing, or cold extrusion.
Full annealing
Full annealing is the process of slowly raising the temperature about 50 ºC (90 ºF) above the Austenitic temperature line A3 or line ACM in the case of Hypoeutectoid steels (steels with < 0.77% Carbon) and 50 ºC (90 ºF) into the Austenite-Cementite region in the case of Hypereutectoid steels (steels with > 0.77% Carbon). It is held at this temperature for sufficient time for all the material to transform into Austenite or Austenite-Cementite as the case may be. It is then slowly cooled at the rate of about 20 ºC/hr (36 ºF/hr) in a furnace to about 50 ºC (90 ºF) into the Ferrite-Cementite range. At this point, it can be cooled in room temperature air with natural convection.
Stress Relief Anneal
Stress Relief Anneal is used to reduce residual stresses in large castings, welded parts and cold-formed parts. Such parts tend to have stresses due to thermal cycling or work hardening. Parts are heated to temperatures of up to 600 - 650 ºC (1112 - 1202 ºF), and held for an extended time (about 1 hour or more) and then slowly cooled in still air.
Spheroidization
Spheroidization is an annealing process used for high carbon steels (Carbon > 0.6%) that will be machined or cold formed subsequently. This is done by one of the following ways:
1. Heat the part to a temperature just below the Ferrite-Austenite line, line A1 or below the Austenite-Cementite line, essentially below the 727 ºC (1340 ºF) line. Hold the temperature for a prolonged time and follow by fairly slow cooling. Or
2. Cycle multiple times between temperatures slightly above and slightly below the 727 ºC (1340 ºF) line, say for example between 700 and 750 ºC (1292 - 1382 ºF), and slow cool. Or
3. For tool and alloy steels heat to 750 to 800 ºC (1382-1472 ºF) and hold for several hours followed by slow cooling.
All these methods result in a structure in which all the Cementite is in the form of small globules (spheroids) dispersed throughout the ferrite matrix. This structure allows for improved machining in continuous cutting operations such as lathes and screw machines. Spheroidization also improves resistance to abrasion.
NORMALIZING
Normalizing is a technique used to provide uniformity in grain size and composition throughout an alloy. The term is often used for ferrous alloys that have been heated above the upper critical temperature and then cooled in open air. When normalising, the steel is heated to a temperature (approx. 20°C to 50°C) above the upper transformation point Ac3, for hypereutectoid steels above Ac1, and is then cooled in static air. It is used to achieve an even, fine-grained microstructure.
Normalizing is the process of raising the temperature to over 60 º C (108 ºF), above line A3 or line ACM fully into the Austenite range. It is held at this temperature to fully convert the structure into Austenite, and then removed form the furnace and cooled at room temperature under natural convection. This results in a grain structure of fine Pearlite with excess of Ferrite or Cementite. The resulting material is soft; the degree of softness depends on the actual ambient conditions of cooling. This process is considerably cheaper than full annealing since there is not the added cost of controlled furnace cooling.
For hypoeutectoid steels, the microstructure consists of perlite and ferrite and for hypereutectoid steels of perlite and cementite. The higher the heating and cooling speeds, the finer the grains in the microstrurture become, providing that the transformation during cooling takes place in the perlite stage.
Through normalising, an uneven and coarse grained microstructure which has come about during hot forming can be eliminated. In addition, for steels with C-contents of less than 0.5% which transform easily, the adjustment to a perlitic-ferritic-microstructure with largely even distribution leads to good machining properties. Normalizing is a type of heat treatment applicable to ferrous metals only. It differs from annealing in that the metal is heated to a higher temperature and then removed from the furnace for air cooling.
Normalized steels are harder and stronger than an-nealed steels. In the normalized condition, steel is much tougher than in any other structural condition. Parts subjected to impact and those that require maximum toughness with resistance to external stress are usually normalized. In normalizing, the mass of metal has an influence on the cooling rate and on the resulting structure. Thin pieces cool faster and are harder after normal-izing than thick ones. In annealing (furnace cooling), the hardness of the two are about the same.









 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope