Electroplating is the process of using an electrical current to coat an electrically conductive object with a thin layer of metal. Copper plating is the process in which a layer of copper is deposited on the item to be plated by using an electric current.
Three basic types of processes are commercially available based upon the complexing system utilized :
- alkaline-(several modifications of cyanide and non-cyanide) complexed bath
- acid-(sulfate and fluoborate) complexed bath
- mildly alkaline-(pyro phosphate) complexed bath
With a higher current, hydrogen bubbles will form on the item to be plated, leaving surface imperfections. Often various other chemicals are added to improve plating uniformity and brightness. Without some form of additive, it is almost impossible to obtain a smooth plated surface. These additives can be anything from dish soap to proprietary compounds.
Copper plating is a coating of copper metal on another material, often other metals. Plating is designed to increase durability, strength, or visual appeal, and copper plating specifically is often used to improve heat and electrical conductivity. Copper plating is seen most often in wiring and cookware.
Occasionally, copper plating is used for decorative purposes, giving objects a brassy look. Copper plating is more often used, however, for electrical wires since copper conducts heat extremely well. Additionally, many circuit boards are plated with copper.
Since copper is an exceptional heat conductor, copper plating is popular in cookware as well. The speed with which copper heats allows for even surface heat, and, therefore, allows for more even cooking. Professional chefs generally used solid copper cookware, usually lined with steel for increased durability, but these are expensive and not generally in the budget of a hobby cook. Plated pots and pans are usually aluminum or steel plated with copper. This plated cookware still allows for the benefits of copper heating without the expense of the pure copper alternatives.
Chemical
- electrolyte solution (200 g CuSO4· 5H2O + 25.0 mL concentrated H2SO4 solution in enough distilled or deionized water to make l.00 L of solution)*
- pre-1983 pennies
- vinegar
- NaCl
Equipments
- power supply (6.0-9.0 volts, 0.60-1.0 amps)*
- connecting wires with alligator clips
- 16-18 gauge copper wire
- 250-mL beaker
- cardboard square (approx. 15 cm on a side)
- ammeter (optional)
Procedure
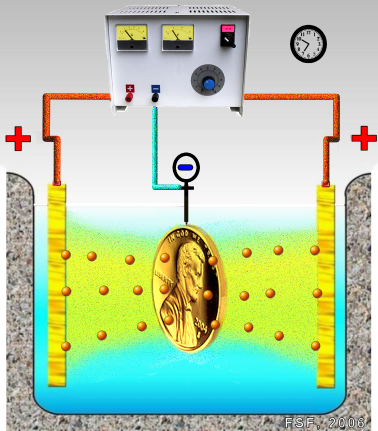
- Pour 200 mL of the electrolyte solution into the beaker.
- Attach connecting wires with alligator clips to the terminals of the power supply.
- Clean the pennies with a mixture of 3 g NaCl and 15 mL vinegar, rinse and dry.
- Tightly wrap one end of a 10-cm length of copper around each penny, leaving 5-6 cm of wire free.
- Mass each penny-copper wire assembly and record the masses.
- Push the free end of each wire through the cardboard square and place the square over the beaker so that the penny “electrodes” are immersed in the electrolyte solution as illustrated below. Note: the two electrode assemblies must not touch.
Disposal
Pennies used in this experiment should not be reused as currency. Solids may be discarded with other solid waste. Electrolyte solution should be stored for re-use; if it becomes necessary to dispose of the solution, it should be flushed down the drain with plenty of water.
Discussion
As copper is plated out at the cathode (negative electrode), copper goes into solution at the anode (positive electrode) as copper(II) ions, maintaining a constant concentration of copper(II) ions in the electrolytic solution.
cathode: Cu2+(aq) + 2 e- Cu(s)
anode: Cu(s) Cu2+(aq) + 2 e-
Commercial plating is done very slowly in order to obtain a smooth, even coating of the plated metal. Although this experiment does not produce plating of commercial quality, it gives students the opportunity to study the chemistry of an important commercial process. This general method is also used in purifying copper. A small cathode of pure copper is used with a larger anode of impure copper. As the electrolytic cell operates, pure copper is transferred to the cathode. Students should be introduced to Faraday’s law before doing this experiment. From this law, students will note that 2 x 96,485 coulombs of charge are required to produce one mole of copper from copper(II) ion.
If an ammeter reading is taken, the number of coulombs that actually passed through the electrolytic cell can be calculated by using the formula : q = It, where q is the charge in coulombs, I is the current in amperes, and t is the time in seconds. From the coulombs of charge that pass through the cell, students can calculate the theoretical number of moles of copper that should have plated out and compare this to the actual number of moles that were plated out. If one assumes that the theoretical yield is equal to the actual yield, the atomic mass of copper can be calculated.
If an ammeter reading is not taken, students can compare the changes in mass of the two electrodes and from the number of moles of copper plated, calculate the number of coulombs of charge passed through the cell and the average current through the cell. Copper is an active metal and so difficult to plate onto a passivated surface, making direct plating of iron based metals difficult. Such surfaces often require a nickel strike base coat for the copper to adhere to.
The plating seen in chemistry classes, often obtained with a coin and copper sulphate bath, is in fact deposition as opposed to plating. Subjecting the surface to any wear causes the unstuck deposit to come away. Running such a bath for longer periods, one can often see the grainy like texture of deposition as opposed to the smooth surface of plating. Commercial platers often use a copper cyanide-based solution to ensure a high level of copper remains in solution. These solutions are inherently dangerous due to the highly toxic nature of cyanide.
Copper plating is often applied by a process called electroplating. Electroplating is simple enough to be done at home, but can be dangerous so is not recommending for the inexperienced. Simple setups of electroplating are often used in high school science demonstrations, but nickel rather than copper is most often used as the plating substance.
You might also like
| Electroplating Electroplating is the process of... | Nickel and Nickel Alloys Nickel is a chemical element, with... | Advanced Ceramics A ceramic is an inorganic, nonmetallic... | Metal Spraying Metal spraying is spraying hot metal... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope