A superconductor is an element or metallic alloy which, when cooled to near absolute zero, dramatically lose all electrical resistance. In principle, superconductors can allow electrical current to flow without any energy loss (although, in practice, an ideal superconductor is very hard to produce). This type of current is called a supercurrent.
 Liquid nitrogen is also used to cool certain metals to the point where they become superconductors. Superconductors conduct electricity (electrons) without any resistance.
Liquid nitrogen is also used to cool certain metals to the point where they become superconductors. Superconductors conduct electricity (electrons) without any resistance.
Superconductors, materials that have no resistance to the flow of electricity, are one of the last great frontiers of scientific discovery. Not only have the limits of superconductivity not yet been reached, but the theories that explain superconductor behavior seem to be constantly under review. In 1911 superconductivity was first observed in mercury by Dutch physicist Heike Kamerlingh Onnes of Leiden University (shown above). When he cooled it to the temperature of liquid helium, 4 degrees Kelvin (-452F, -269C), its resistance suddenly disappeared. The Kelvin scale represents an “absolute” scale of temperature. Thus, it was necessary for Onnes to come within 4 degrees of the coldest temperature that is theoretically attainable to witness the phenomenon of superconductivity. Later, in 1913, he won a Nobel Prize in physics for his research in this area.
 Superconductors, materials that have no resistance to the flow of electricity
Superconductors, materials that have no resistance to the flow of electricity
The next great milestone in understanding how matter behaves at extreme cold temperatures occurred in 1933. German researchers Walther Meissner (above left) and Robert Ochsenfeld (above right) discovered that a superconducting material will repel a magnetic field (below graphic).
A magnet moving by a conductor induces currents in the conductor. This is the principle on which the electric generator operates. But, in a superconductor the induced currents exactly mirror the field that would have otherwise penetrated the superconducting material - causing the magnet to be repulsed. This phenomenon is known as strong diamagnetism and is today often referred to as the “Meissner effect” (an eponym). The Meissner effect is so strong that a magnet can actually be levitated over a superconductive material.
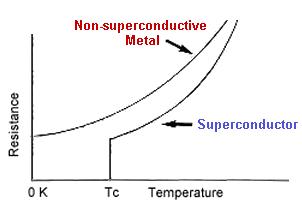 Superconductor versus Non-Superconductor
Superconductor versus Non-Superconductor
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum mechanical phenomenon.
It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor as it transitions into the superconducting state. The occurrence of the Meissner effect indicates that superconductivity cannot be understood simply as the idealization of perfect conductivity in classical physics.
 Superconductivity is a phenomenon observed in several metals and ceramic materials. When these materials are cooled to temperatures ranging from near absolute zero ( 0 degrees Kelvin, -273 degrees Celsius) to liquid nitrogen temperatures ( 77 K, -196 C), their electrical resistance drops with a jump down to zero.
Superconductivity is a phenomenon observed in several metals and ceramic materials. When these materials are cooled to temperatures ranging from near absolute zero ( 0 degrees Kelvin, -273 degrees Celsius) to liquid nitrogen temperatures ( 77 K, -196 C), their electrical resistance drops with a jump down to zero.
Superconductivity is a phenomenon observed in several metals and ceramic materials. When these materials are cooled to temperatures ranging from near absolute zero (-459 degrees Fahrenheit, 0 degrees Kelvin, -273 degrees Celsius) to liquid nitrogen temperatures (-321 F, 77 K, -196 C), they have no electrical resistance. The temperature at which electrical resistance is zero is called the critical temperature (Tc) and varies with the individual material. For practical purposes, critical temperatures are achieved by cooling materials with either liquid helium or liquid nitrogen. The following table shows the critical temperatures of various superconductors:
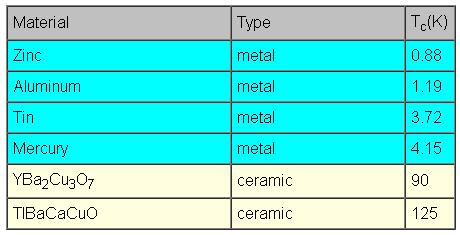 The critical temperatures of various superconductors
The critical temperatures of various superconductors
How do electrons travel through superconductors with no resistance? Lets’s look at this more closely. The atomic structure of most metals is a lattice structure, much like a window screen in which the intersection of each set of perpendicular wires is an atom.
Metals hold on to their electrons quite loosely, so these particles can move freely within the lattice — this is why metals conduct heat and electricity very well. As electrons move through a typical metal in the normal state, they collide with atoms and lose energy in the form of heat. In a superconductor, the electrons travel in pairs and move quickly between the atoms with less energy loss.
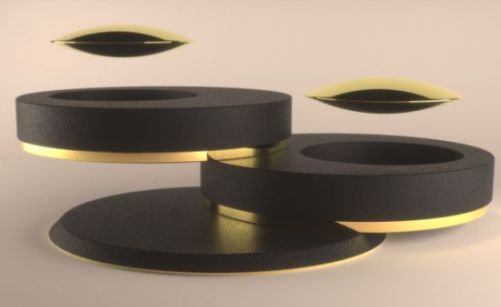 Levitating Superconductor Speakers
Levitating Superconductor Speakers
As a negatively-charged electron moves through the space between two rows of positively-charged atoms (like the wires in a window screen), it pulls inward on the atoms. This distortion attracts a second electron to move in behind it. This second electron encounters less resistance, much like a passenger car following a truck on the freeway encounters less air resistance. The two electrons form a weak attraction, travel together in a pair and encounter less resistance overall. In a superconductor, electron pairs are constantly forming, breaking and reforming, but the overall effect is that electrons flow with little or no resistance.
The future of superconductivity research is to find materials that can become superconductors at room temperature. Once this happens, the whole world of electronics, power and transportation will be revolutionized.
Do you need book`s references about Superconductor?
You might also like
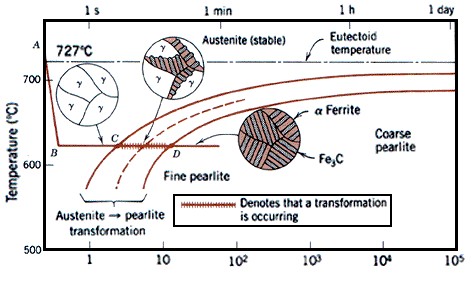
| Types of Materials Metals: Metals are elements... | Shape Memory Alloy "Animated lamp" designed by Romolo Stanco... | Fe-Fe3C T-T-T Diagram Fe-Fe3C T-T-T Diagram, Adapted from... | Phase Diagram of Steel Fe-Fe3C Phase Diagram, Materials Science... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope