Copper Alloys
Copper is one of the most useful metals known to man, and it was one of the first to be utilized. Copper is a reddish-yellow material and is extremely ductile. Copper has a face-centered-cubic (fcc) crystal structure and has the second best electrical conductivity of the metals, second only to silver compared to which it has a conductivity of 97%.

Copper is one of the most useful metals known to man
The thermal conductivity of copper is very high falling in between silver and gold. There are almost 400 different copper alloys depending on the commercial product made likes : rods, plates, sheets, strips, tubes, pipes, extrusions, foils, forgings, wires, and castings from foundries.
 Copper is one of the most useful metals known to man, and it was one of the first to be utilized.
Copper is one of the most useful metals known to man, and it was one of the first to be utilized.
Copper alloys are metal alloys that have copper as their principal component. They have high resistance against corrosion. The best known traditional types are bronze, where tin is a significant addition, and brass, using zinc instead. Both these are imprecise terms, and today the term copper alloy tends to be substituted, especially by museums.
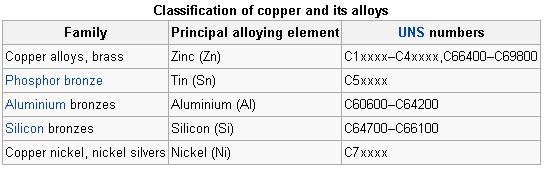
The similarity in external appearance of the various alloys, along with the different combinations of elements used when making each alloy, can lead to confusion when categorizing the different compositions.
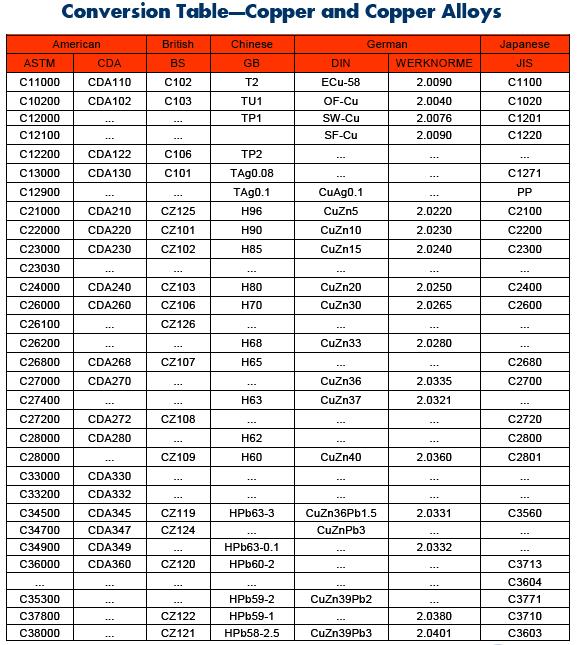
There are as many as 400 different copper and copper-alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys.
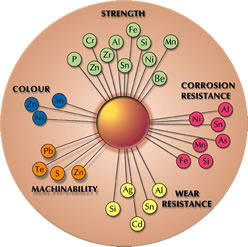
The addition of alloying elements improves certain properties eg tin, aluminium, nickel, iron and silicon are used to improve corrosion resistance, lead is added to improve machinability and silicon is added to improve wear resistance.
Pure copper has the best electrical and thermal conductivity of any commercial metal. Copper forms alloys more freely than most metals and with a wide range of alloying elements to produce the following alloys :
- tin makes tin bronze
- tin and phosphorus makes phosphor bronze
- aluminium makes aluminium bronze
- zinc makes brass
- tin and zinc makes gunmetal
- nickel makes copper-nickel
- nickel and zinc make nickel silver
- beryllium makes beryllium copper.
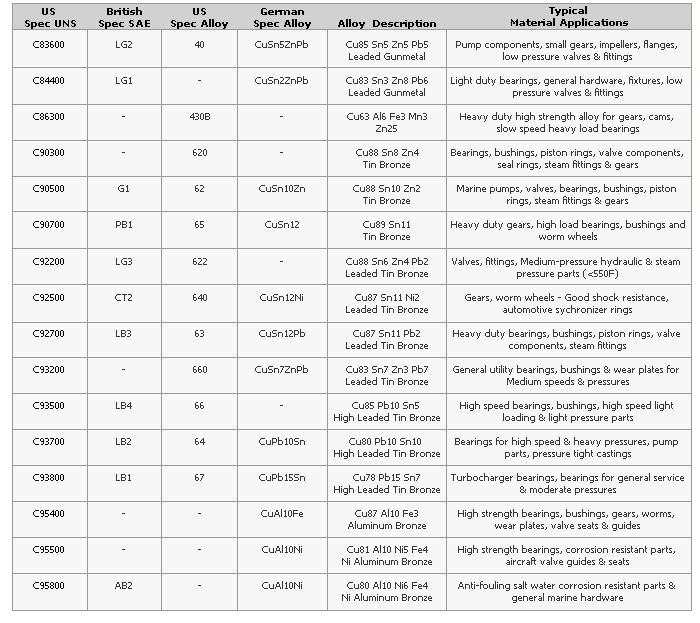 Quick Reference Guide for Copper Alloys
Quick Reference Guide for Copper Alloys
The following table lists the principal alloying element for four of the more common types used in modern industry, along with the name for each type. Historical types, such as those that characterize the Bronze Age, are vaguer as the mixtures were generally variable.

Brass Parts
Brass
A brass is an alloy of copper with zinc. Brasses are usually yellow in color. The zinc content can vary between few % to about 40%; as long as it is kept under 15%, it does not markedly decrease corrosion resistance of copper.

Brass Dragon
Brasses can be sensitive to selective leaching corrosion under certain conditions, when zinc is leached from the alloy (dezincification), leaving behind a spongy copper structure.
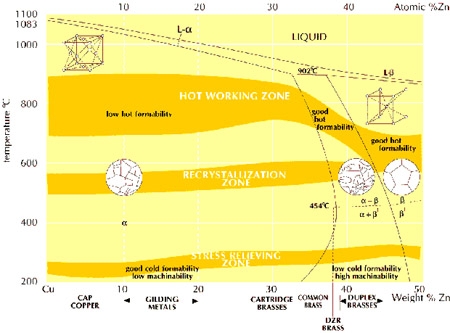
The diagram shows the structure of binary copper-zinc alloys when fully in equilibrium, indicating the preferred temperatures for stress relieving, full annealing and hot working. The differences in the properties of the phases are partly due to the fact that alpha brass has a face-centered cubic structure while beta brass is body-centered cubic.
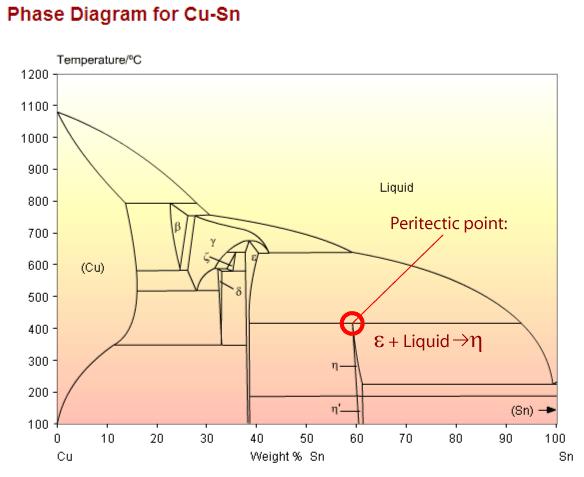
Copper tin phase diagram showing a peritectic point. The peritectic reaction is an important example of a microstructural transformation. Sn – 21wt.%Cu exhibits this transformation from a solid phase and a liquid phase to a different, solid phase.

Bronze Medal

SonyEricsson Bronze casing
Bronze
A bronze is an alloy of copper and other metals, most often tin, but also aluminium and silicon.
- Aluminium bronzes are alloys of copper and aluminium. The content of aluminium ranges mostly between 5-11%. Iron, nickel, manganese and silicon are sometimes added. They have higher strength and corrosion resistance than other bronzes, especially in marine environment, and have low reactivity to sulfur compounds. Aluminium forms a thin passivation layer on the surface of the metal.
- Bell metal
- Phosphor bronze
- Nickel bronzes, e.g. nickel silver and cupronickel
- Speculum metal
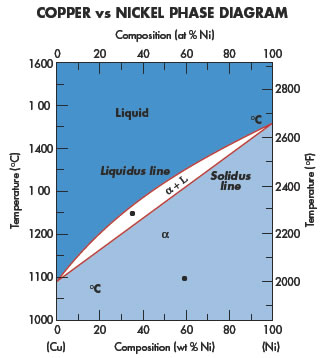
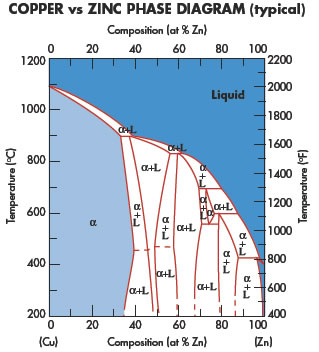
Copper application areas cover a wide variety of different disciplines.
- Architecture, both exterior and interior
- Automotive, Copper is an essential component of many of the latest design elements in today’s cars.
- Electrical Copper’s high conductivity makes it the ideal material in a wide variety of electical applications
- Electrical Energy, Efficiency Power QualityBuilding WireTube, Pipe & FittingsCopper tube is the highest quality material available today for a variety of building applications including plumbing, fire sprinklers.
- Fuel Gas, Copper tube is an excellent choice for natural gas piping systems.
- Industrial, Copper serves as an essential material in a vast number of industries including electronics.
- Seawater Copper’s unique properties make it ideal for many applications in the harsh environments of marine
- Machined Products, Copper alloy rod and bar products.
- Telecommunications, Communications are the backbone of today’s fast-paced businesses, and copper wiring is at the core of those systems.








 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope