A study of the microstructure of all steels usually starts with the metastable iron-carbon (Fe-C) binary phase diagram (Figure 1). It provides an invaluable foundation on which to build knowledge of both carbon steels and alloy steels, as well as a number of various heat treatments they are usually subjected to (hardening, annealing, etc).
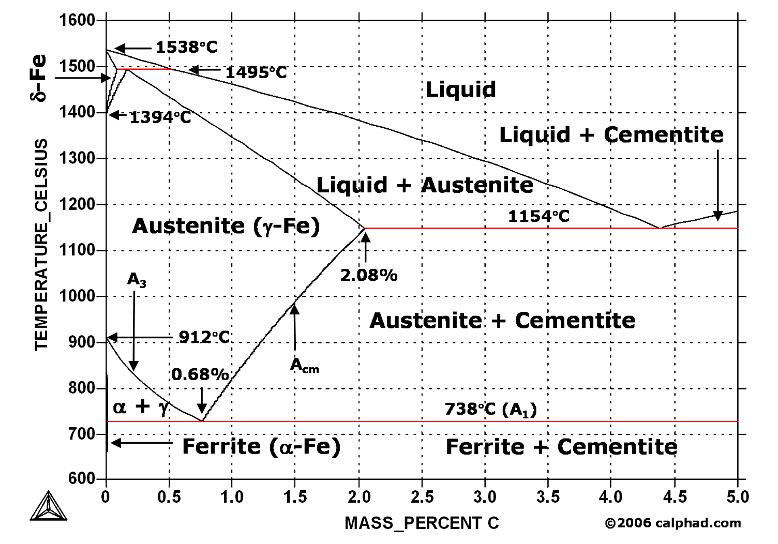 Figure 1. Iron Carbon Phase Diagram
Figure 1. Iron Carbon Phase Diagram
At the low-carbon end of the metastable Fe-C phase diagram, we distinguish ferrite (alpha-iron), which can at most dissolve 0.028 wt. % C at 738 °C, and austenite (gamma-iron), which can dissolve 2.08 wt. % C at 1154 °C. The much larger phase field of gamma-iron (austenite) compared with that of alpha-iron (ferrite) indicates clearly the considerably grater solubility of carbon in gamma-iron (austenite), the maximum value being 2.08 wt. % at 1154 °C. The hardening of carbon steels, as well as many alloy steels, is based on this difference in the solubility of carbon in alpha-iron (ferrite) and gamma-iron (austenite).
You might also like
| Metallurgy Glossary Metallurgy Glossary Activity: A function... | Phase Diagrams Fe-Mn, Fe-Co, Fe-Mo In pure iron, the A4 (1394 °C) and... | Iron-Carbon Phases Influence of Temperature on Crystal Structure The... | Cast Iron Cast iron is derived from pig iron,... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope