Yellowcake (also called urania) is a kind of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore forming a coarse powder which has a pungent odour, is insoluble in water and contains about 80% uranium oxide, which melts at approximately 2878 °C. Although uranium is one of the densest metals on Earth, yellowcake is relatively light, with a density approximately that of elemental sulfur.
 Yellowcake - Uranium
Yellowcake - Uranium
Uranium is present in the Earth’s crust at an average concentration of 2 parts per million. Acidic rocks with high silicate, such as granite, have higher than average concentrations of uranium, while sedimentary and basic rocks have lower than average concentrations. Uranite or pitchblende (U3O8), the most common uranium-containing ores, are mixtures of UO2 (basic) and UO3 (amphoteric) oxides. The richest ores are found in the western United States, Canada, Australia, South Africa, the former Soviet Union, and Zaire (the former Belgian Congo). The concentration of U3O8 in ores can vary from 0.5% in Australian ores to 20% in Canadian ores.
As we have seen, the discovery of fission led to two potential routes to weapons for the scientists in America in the 1940′s. Each required fissile isotopes that would release energy and neutrons quickly. The scientists could isolate plutonium formed by bombarding of U-238 with neutrons or they could attempt to separate U-235 from natural uranium ore. But both approaches required uranium.
The ore is first crushed to a fine powder by passing raw uranium ore through crushers and grinders to produce “pulped” ore. This is further processed with concentrated acid, alkaline, or peroxide solutions to leach out the uranium. Yellowcake is what remains after drying and filtering. The yellowcake produced by most modern mills is actually brown or black, not yellow, the name comes from the color and texture of the concentrates produced by early mining operations.
 Yellowcake - Urania
Yellowcake - Urania
Initially, the compounds formed in yellowcakes were not identified, in 1970, the U.S. Bureau of Mines still referred to yellowcakes as the final precipitate formed in the milling process and considered it to be ammonium diuranate or sodium diuranate. The compositions were variable and depended upon the leachant and subsequent precipitating conditions. Among the compounds identified in yellowcakes include: uranyl hydroxide, uranyl sulfate, sodium para-uranate, and uranyl peroxide, along with various uranium oxides. Modern yellowcake typically contains 70 to 90 percent triuranium octoxide (U3O8) by weight. Other oxides such as uranium dioxide (UO2) and uranium trioxide (UO3) exist.
Yellowcake is used in the preparation of uranium fuel for nuclear reactors, for which it is smelted into purified UO2 for use in fuel rods for pressurized heavy-water reactors and other systems that use natural unenriched uranium. Purified uranium metal (not the uranium oxide) can also be enriched in the isotope U-235. In this process, the uranium is combined with fluorine to form uranium hexafluoride gas (UF6). Next, that undergoes isotope separation through the process of gaseous diffusion, or in a gas centrifuge.
 Yellowcake is used in the preparation of uranium fuel for nuclear reactors
Yellowcake is used in the preparation of uranium fuel for nuclear reactors
This can produce low-enriched uranium containing up to 20 percent U-235 that is suitable for use in large civilian electric-power reactors. With further processing one obtains highly enriched uranium, containing 20% or more U-235, that is suitable for use in compact nuclear reactors—usually used to power naval warships and submarines. Further processing can yield weapons-grade uranium with U-235 levels usually above 90%, suitable for nuclear weapons. However, since the collapse of the Soviet Union, there is a worldwide surplus of highly-enriched uranium, and Russian HEU is diluted to LEU for power reactors.
 The Real Yellow Cake ! Hmm…Delicious !! NOT Yellowcake !
The Real Yellow Cake ! Hmm…Delicious !! NOT Yellowcake !
The overall objective of uranium extraction chemistry is the preparation of U3O8, called yellow cake. Extraction of uranium is often difficult and the metallurgical procedures vary with the geological environment of the ore. The ore is first crushed and ground to liberate mineral particles. The amphoteric oxide is then leached with sulfuric acid.
Basic Reaction
UO3(s) + 2H+(aq) -> UO22+(aq) + H2O
UO22+(aq) + 3SO42-(aq) -> UO2(SO4)34-(aq)
The basic oxide is converted by a similar process to that of a water soluble
UO2(CO3)34-(aq) ion.
Two methods are used to concentrate and purify the uranium: ion exchange and solvent extraction. Solvent extraction, the more common method, uses tertiary amines in an organic kerosene solvent in a continuous process.
First the amines, R3N, react with sulfuric acid :
2 R3N(org) + H2SO4(aq) -> (R3NH)2SO4(org)
Then the amine sulfate extracts the uranyl ions into the organic phase while the impurities remain in the aqueous phase. In the case of the uranyl sulfate ion, the following reactions occur :
(R3NH)2SO4(org) + UO2(SO4)34-(aq) -> (R3NH)4UO2(SO4)3(org) + 2SO42-(aq)
The solvents are removed by evaporating in a vacuum and ammonium diuranate, (NH4)2U2O7, is precipitated by adding ammonia to neutralize the solution. The diuranate is then heated to yield a purified, solid U3O8, known as yellow cake.
Refining and converting U3O8 to UF 6
At the refinery, the yellow cake is dissolved in nitric acid. The resulting solution of uranium nitrate, UO2(NO3)2· 6H2O, is fed into a continuous solvent extraction process. The uranium is extracted into an organic phase (kerosene) with tributyl phosphate, and the impurities remain again in the aqueous phase. After this purification, the uranium is washed out of the kerosene with dilute nitric acid and concentrated by evaporation to pure UO2(NO3)26H2O. Heating yields pure UO3. The initial separation and refining processes generate large volumes of acid and organic waste.
It is necessary to enrich the U-235 isotope concentration from its natural composition of 0.7% for use in either reactors or bombs. Reactor grade uranium contains from 3.5 to 4.0% U-235, while the Hiroshima uranium bomb contained more than 80% of the lighter U-235. The process used for enrichment involves gaseous diffusion and thus the uranium must be converted to a gaseous compound, uranium hexafluoride (UF6).
Conversion to the hexafluoride involves the following sequence of reactions.
The UO3 is reduced with hydrogen in a kiln :
UO3(s) + H2(g) -> UO2(s) + H2O(g)
The uranium dioxide is then reacted with hydrogen fluoride to form uranium tetrafluoride :
UO2(s) + 4HF(g) -> UF4(s) + 4H2O(g)
The tetrafluoride is then fed into a fluidized bed reactor and reacted with gaseous fluorine to obtain the hexafluoride :
UF4(s) + F2(g) -> UF6(g)
The hexafluoride is now suitable feedstock for the gaseous diffusion process.
Production of uranium metal
Uranium metal is produced by reducing the uranium tetrafluoride with either calcium or magnesium, both active group IIA metals that are excellent reducing agents.
UF4(s) + 2Ca(s) -> U(s) + 2CaF2(s)
This reduction may be done before or after the enrichment process, depending on the intended use of the uranium. Reactors use both enriched (3 to 5% U-235) uranium metal and uranium oxide as fuel while weapons use more highly enriched uranium (up to 90% U-235).
You might also like
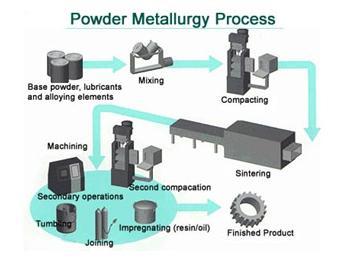
| Dysprosium Dysprosium Dysprosium is a chemical element... | Tungsten In 1779 Peter Woulfe deduced the existence... | Powder Metallurgy Powder Metallurgy Process, source : https://www.themetalcasting.com/ Powder... | Fracture Mechanics Concepts The basis of a fracture mechanics safety... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope