Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal. Most silver is produced as a byproduct of copper, gold, lead, and zincrefining.
 Silver Coin
Silver Coin
Among metals, pure silver has the highest thermal conductivity (the nonmetal diamond and superfluid helium II are higher) and one of the highest optical reflectivities. Aluminium slightly outdoes silver in parts of the visible spectrum, and silver is a poor reflector of ultraviolet light.
 A nugget of silver
A nugget of silver
Silver also has the lowest contact resistance of any metal. Silver has been known since ancient times. Silver mining was a driving force in the settlement of western North America, with major booms for silver and associated minerals (lead, mostly) in the galena ore silver is most commonly found in. Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag, with 107Ag being the most abundant (51.839% natural abundance).
Silver metal dissolves readily in nitric acid (HNO3) to produce silver nitrate (AgNO3), a transparent crystalline solid that is photosensitive and readily soluble in water. Silver metal does not react with sulfuric acid, which is used in jewelry-making to clean and remove copper oxide firescale from silver articles after silver soldering or annealing. However, silver reacts readily with sulfur or hydrogen sulfide H2S to produce silver sulfide, a dark-colored compound familiar as the tarnish on silver coins and other objects. Silver sulfide also forms silver whiskers when silver electrical contacts are used in an atmosphere rich in hydrogen sulfide.
 Silver Ring
Silver Ring
Silver chloride (AgCl) is precipitated from solutions of silver nitrate in the presence of chloride ions, and the other silver halides used in the manufacture of photographic emulsions are made in the same way, using bromide or iodide salts. Silver oxide (Ag2O), produced when silver nitrate solutions are treated with a base, is used as a positive electrode (anode) in watch batteries. Silver carbonate (Ag2CO3) is precipitated when silver nitrate is treated with sodium carbonate (Na2CO3).
 Silver Block for Invesment
Silver Block for Invesment
Silver fulminate (AgONC), a powerful, touch-sensitive explosive used in percussion caps, is made by reaction of silver metal with nitric acid in the presence of ethanol (C2H5OH). Another dangerously explosive silver compound is silver azide (AgN3), formed by reaction of silver nitrate with sodium azide (NaN3). Alkaline solutions of silver nitrate can be reduced to silver metal by reducing sugars such as glucose, and this reaction is used to silver glass mirrors and the interior of glass Christmas ornaments.
Silver halides are soluble in solutions of sodium thiosulfate (Na2S2O3) which is used as a photographic fixer, to remove excess silver halide from photographic emulsions after image development. Silver forms cyanide complexes (silver cyanide) that are soluble in water in the presence of an excess of cyanide ions. Silver cyanide solutions are used in electroplating of silver.Ores include argentite (Ag2S), chlorargyrite (AgCl) which includes horn silver, and pyrargyrite (Ag3SbS3).
 Silver at Electronic Devices
Silver at Electronic Devices
Many well known uses of silver involve its precious metal properties, including currency, decorative items and mirrors. Silver, in the form of electrum (a gold-silver alloy), was coined to produce money around 700 BC by the Lydians. Later, silver was refined and coined in its pure form. Many nations used silver as the basic unit of monetary value. In the modern world, silver bullion has the ISO currency code XAG. Jewelry and silverware are traditionally made from sterling silver (standard silver), an alloy of 92.5% silver with 7.5% copper.
 Silver Ores
Silver Ores
In the US, only an alloy consisting of at least 90.0% fine silver can be marketed as “silver” (thus frequently stamped 900). Sterling silver (stamped 925) is harder than pure silver, and has a lower melting point (893 °C) than either pure silver or pure copper. Britannia silver is an alternative, hallmark-quality standard containing 95.8% silver, often used to make silver tableware and wrought plate. Sterling silver jewelry is often plated with a thin coat of .999 fine silver to give the item a shiny finish. Silver jewelry can also be plated with rhodium (for a bright, shiny look) or gold.
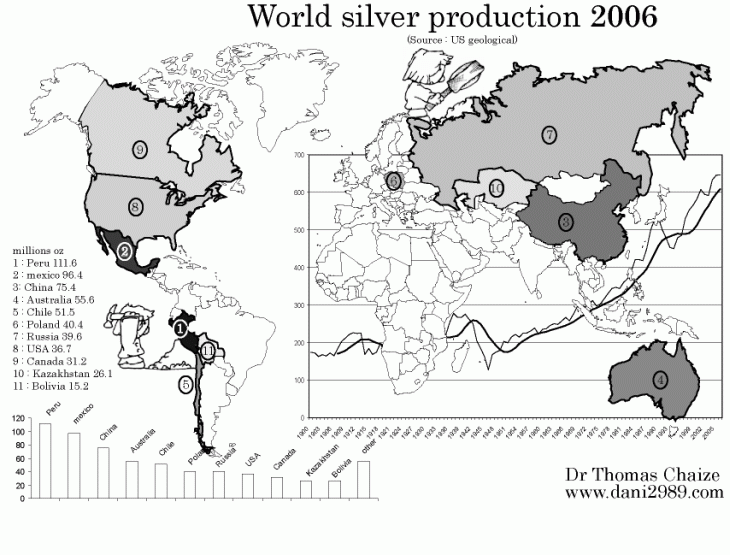 World Silver
World Silver
Silver is a constituent of almost all colored carat gold alloys and carat gold solders, giving the alloys paler color and greater hardness. White 9 carat gold contains 62.5% silver and 37.5% gold, while 22 carat gold contains up to 91.7 gold and 8.4% silver or copper or a mixture of both. Photography used 30.98% of the silver consumed in 1998 in the form of silver nitrate and silver halides. Some electrical and electronic products use silver for its superior conductivity, even when tarnished. As an additional example, printed circuits and RFID antennas can be made using silver paints, and computer keyboards use silver electrical contacts. Silver cadmium oxide is used in high voltage contacts because it can withstand arcing.Though debatable, many hi-fi enthusiasts believe silver wires improve sound quality.
Silver and silver alloys are used in the construction of high quality musical wind instruments of many types. Flutes, in particular, are commonly constructed of silver alloy or silver plated, both for appearance and for the frictional surface properties of silver.Silver’s catalytic properties make it ideal for use as a catalyst in oxidation reactions, for example, the production of formaldehyde from methanol and air by means of silver screens or crystallites containing a minimum 99.95 weight-percent silver.
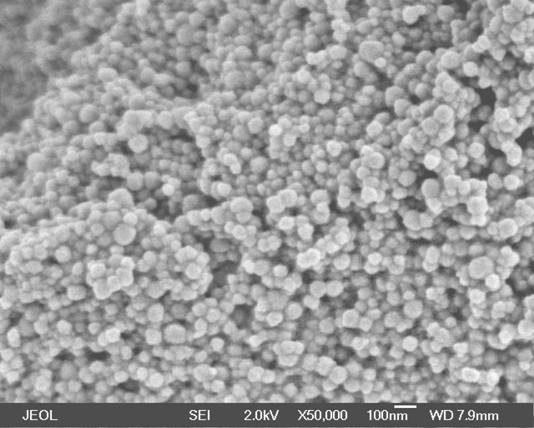
The antimicrobial properties of silver stem from the chemical properties of its ionized form, Ag+. Silver nitrate solution use continued, then was largely replaced by silver sulfadiazine cream (SSD cream), which generally became the “standard of care” for the antibacterial and antibiotic treatment of serious burns until the late 1990s. Now, other options, such as silver-coated dressings (activated silver dressings), are used in addition to SSD cream. There has been renewed interest in silver as a broad-spectrum antimicrobial agent. In 2009, the antibacterial action of silver electrodes was found to be greatly improved if the electrodes were covered with silver nanorods.
The University of Missouri has found silver nanoparticles threaten benign bacteria which extract ammonia from sewage treatment systems. Silver is commonly used in catheters. This meta-analysis clarifies discrepant results among trials of silver-coated urinary catheters by revealing silver alloy catheters are significantly more effective in preventing urinary tract infections than are silver oxide catheters. Various silver compounds, devices to make homeopathic solutions and colloidal silver suspensions are sold as remedies for numerous conditions. Silver has been known since ancient times. In certain circumstances, Islam permits Muslim men to wear silver jewelry. Muhammad himself wore a silver signet ring.
During the war many electrical connectors and switches were silver plated. Since silver can replace tin in solder at a lower volume, a large amount of tin was freed up for other uses by substituting government silver. Silver was used in nickels during the war to save that metal for use in steel alloys.
You might also like
| Dysprosium Dysprosium Dysprosium is a chemical element... | What is Gold? Gold is a chemical element with the symbol... | Silver Nanoparticle What is Silver Nanoparticles ? Silver nanoparticles... | Nickel and Nickel Alloys Nickel is a chemical element, with... |



 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope