Diamond - The Special Ceramic Material
Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions. Diamond has remarkable optical characteristics. Several non-diamond materials, which include cubic zirconia and silicon carbide and are often called diamond simulants, resemble diamond in appearance and many properties. Special gemological techniques have been developed to distinguish natural and synthetic diamonds and diamond simulants. Diamond has a hardness of 10 (hardest) on this scale. Diamond’s hardness has been known since antiquity, and is the source of its name.
 Diamond
Diamond
Diamond hardness depends on its purity, crystalline perfection and orientation: hardness is higher for flawless, pure crystals oriented to the <111> direction (along the longest diagonal of the cubic diamond lattice). Therefore, whereas it might be possible to scratch some diamonds with other materials, such as boron nitride, the hardest diamonds can only be scratched by other diamonds and nanocrystalline diamond aggregates.
The hardness of diamond contributes to its suitability as a gemstone. It is possible to treat regular diamonds under a combination of high pressure and high temperature to produce diamonds that are harder than the diamonds used in hardness gauges. This property can be utilized to extract diamonds using oil when making synthetic diamonds. So acid and alkali can be used to refine synthetic diamonds.
 A 603 Karat Diamond comes from the Letseng Mine in Lesotho - South Africa- named the “Lesotho Promise”
A 603 Karat Diamond comes from the Letseng Mine in Lesotho - South Africa- named the “Lesotho Promise”
Colors in diamond originate from lattice defects and impurities. Nitrogen is by far the most common impurity found in gem diamonds and is responsible for the yellow and brown color in diamonds. Boron is responsible for the blue color. Color in diamond has two additional sources : irradiation (usually by alpha particles), that causes the color in green diamonds and plastic deformation of the diamond crystal lattice. Colored diamonds contain impurities or structural defects that cause the coloration, while pure or nearly pure diamonds are transparent and colorless. Most diamond impurities replace a carbon atom in the crystal lattice, known as a carbon flaw. Diamonds of a different color, such as blue, are called fancy colored diamonds, and fall under a different grading scale.
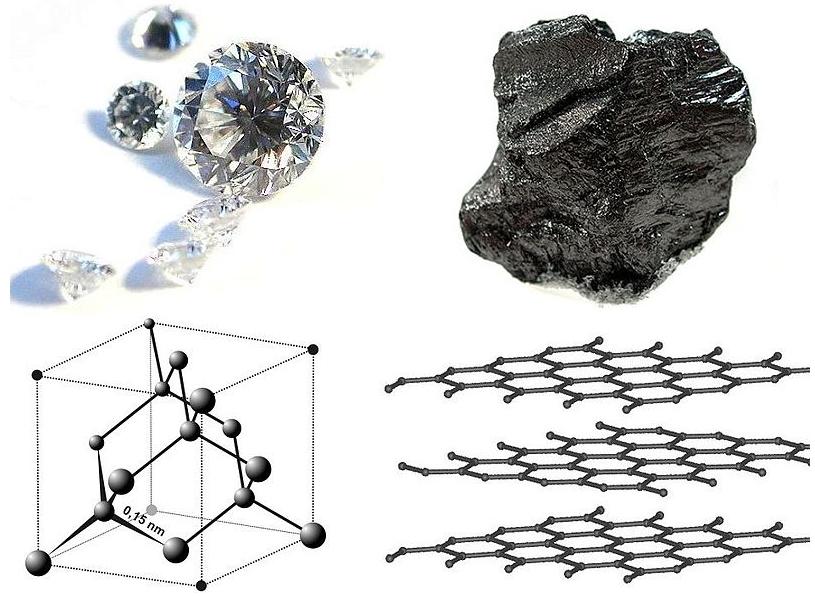 Diamond and graphite are two allotropes of carbon : pure forms of the same element that differ in structure
Diamond and graphite are two allotropes of carbon : pure forms of the same element that differ in structure
Diamonds can be identified by their high thermal conductivity. Diamonds can scratch other diamonds, but this can result in damage to one or both stones. Diamonds also possess an extremely high refractive index and fairly high dispersion. Long residence in the cratonic lithosphere allows diamond crystals to grow larger.
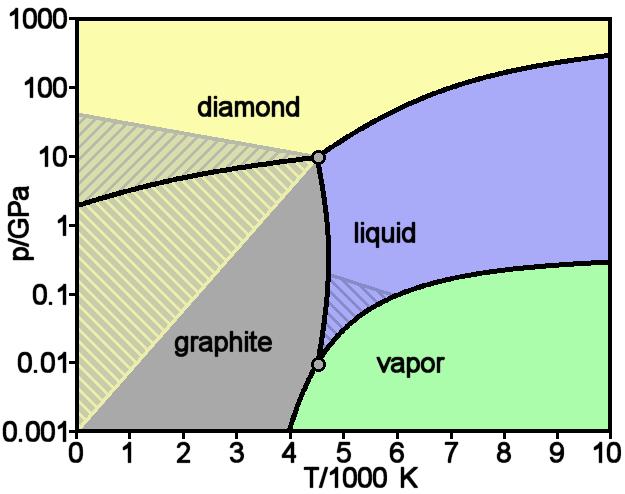 Theoretically predicted phase diagram of carbon
Theoretically predicted phase diagram of carbon
Not all diamonds found on Earth originated here. Diamonds can also form under other naturally occurring high-pressure conditions. Not all pipes contain diamonds, and even fewer contain enough diamonds to make mining economically viable.
 Diamond is the hardest natural material known
Diamond is the hardest natural material known
A volcanic pipe containing diamonds is known as a primary source of diamonds. Only a very small fraction of the diamond ore consists of actual diamonds. Diamonds sold through this process are known as conflict diamonds or blood diamonds. Major diamond trading corporations continue to fund and fuel these conflicts by doing business with armed groups. In response to public concerns that their diamond purchases were contributing to war and human rights abuses in central and western Africa, the United Nations, the diamond industry and diamond-trading nations introduced the Kimberley Process in 2002.
 Diamonds’ chemical property is very stable
Diamonds’ chemical property is very stable
The Kimberley Process aims to ensure that conflict diamonds do not become intermixed with the diamonds not controlled by such rebel groups. Conflict diamonds constitute 2–3% of all diamonds traded. Two major flaws still hinder the effectiveness of the Kimberley Process: (1) the relative ease of smuggling diamonds across African borders, and (2) the violent nature of diamond mining in nations that are not in a technical state of war and whose diamonds are therefore considered “clean”.
The Canadian Government has set up a body known as Canadian Diamond Code of Conduct to help authenticate Canadian diamonds. This is a stringent tracking system of diamonds and helps protect the “conflict free” label of Canadian diamonds.
A large trade in gem-grade diamonds exists. One hallmark of the trade in gem-quality diamonds is its remarkable concentration: wholesale trade and diamond cutting is limited to just a few locations; In 2003, 92% of the world’s diamonds were cut and polished in Surat, India.Other important centers of diamond cutting and trading are the Antwerp diamond district in Belgium, where the International Gemological Institute is based, London, the Diamond District in New York City, Tel Aviv, and Amsterdam.
The production and distribution of diamonds is largely consolidated in the hands of a few key players, and concentrated in traditional diamond trading centers, the most important being Antwerp, where 80% of all rough diamonds, 50% of all cut diamonds and more than 50% of all rough, cut and industrial diamonds combined are handled. This makes Antwerp a de facto “world diamond capital”.
Another important diamond center is New York City, where almost 80% of the world’s diamonds are sold, including auction sales. The DeBeers company, as the world’s largest diamond miner holds a dominant position in the industry, and has done so since soon after its founding in 1888 by the British imperialist Cecil Rhodes. De Beers owns or controls a significant portion of the world’s rough diamond production facilities (mines) and distribution channels for gem-quality diamonds. The Diamond Trading Company (DTC) is a subsidiary of De Beers and markets rough diamonds from De Beers-operated mines.
De Beers and its subsidiaries own mines that produce some 40% of annual world diamond production. As a part of reducing its influence, De Beers withdrew from purchasing diamonds on the open market in 1999 and ceased, at the end of 2008, purchasing Russian diamonds mined by the largest Russian diamond company Alrosa. As at January 2011, De Beers states that it only sells diamonds from the following four countries : Botswana, Namibia, South Africa and Canada. Alrosa had to suspend their sales in October 2008 due to the global energy crisis, but the company reported that it had resumed selling rough diamonds on the open market by October 2009. Apart from Alrosa, other important diamond mining companies include BHP Billiton, which is the world’s largest mining company, Rio Tinto Group, the owner of Argyle (100%), Diavik (60%), and Murowa (78%) diamond mines, and Petra Diamonds, the owner of several major diamond mines in Africa.
Further down the supply chain, members of The World Federation of Diamond Bourses (WFDB) act as a medium for wholesale diamond exchange, trading both polished and rough diamonds. WFDB’s additional activities include sponsoring the World Diamond Congress every two years, as well as the establishment of the International Diamond Council (IDC) to oversee diamond grading.
Diamonds can be sold already set in jewelry, or sold unset (“loose”). Brown-colored diamonds have always constituted a significant part of the diamond production, but were considered worthless for jewelry, they were not even assessed on the diamond color scale, and were predominantly used for industrial purposes. As a result of an aggressive marketing campaign, brown diamonds have become acceptable gems. The change was mostly due to the numbers : the Argyle mine, with its 35,000,000 carats (7,000 kg) of diamonds per year, makes about one-third of global production of natural diamonds, 80% of Argyle diamonds are brown.
You might also like
| What is Zirconia? Zirconia - ZrO2 Zirconium dioxide (ZrO2),... | Austenite - Gamma Iron Austenite - a Definition Austenite also... | What is Carbon Nanotubes? Carbon Nanotubes - Application Nanotechnology Carbon... | What is Hybrid Materials? Hybdrid Material - Advance Material Hybrid... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope