Zirconium dioxide (ZrO2), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the rare mineral baddeleyite. The high temperature cubic crystalline form is rarely found in nature as mineral tazheranite (Zr,Ti,Ca)O2 (and a doubtful mineral arkelite). This form, also called cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant.
Zirconia is an extremely refractory material. It offers chemical and corrosion inertness to temperatures well above the melting point of alumina. The material has low thermal conductivity. It is electrically conductive above 600°C and is used in oxygen sensor cells and as the susceptor (heater) in high temperature induction furnaces. With the attachment of platinum leads, nernst glowers used in spectrometers can be made as a light emitting filament which operates in air.
Key Properties of Zirconium Oxide
- Use temperatures up to 2400°C
- High density
- Low thermal conductivity (20% that of alumina)
- Chemical inertness
- Resistance to molten metals
- Ionic electrical conduction
- Wear resistance
- High fracture toughness
- High hardness
Typical Uses of ZrO2
- Precision ball valve balls and seats
- High density ball and pebble mill grinding media
- Rollers and guides for metal tube forming
- Thread and wire guides
- Hot metal extrusion dies
- Deep well down-hole valves and seats
- Powder compacting dies
- Marine pump seals and shaft guides
- Oxygen sensors
- High temperature induction furnace susceptors
- Fuel cell membranes
- Electric furnace heaters over 2000°C in oxidizing atmospheres
Zirconium dioxide is one of the most studied ceramic materials. Pure ZrO2 has a monoclinic crystal structure at room temperature and transitions to tetragonal and cubic at increasing temperatures. The volume expansion caused by the cubic to tetragonal to monoclinic transformation induces very large stresses, and will cause pure ZrO2 to crack upon cooling from high temperatures. Several different oxides are added to zirconia to stabilize the tetragonal and/or cubic phases: magnesium oxide (MgO), yttrium oxide, (Y2O3), calcium oxide (CaO), and cerium(III) oxide (Ce2O3), amongst others.
 Zirconium Oxide
Zirconium Oxide
Zirconia is very useful in its ‘stabilized’ state. In some cases, the tetragonal phase can be metastable. If sufficient quantities of the metastable tetragonal phase is present, then an applied stress, magnified by the stress concentration at a crack tip, can cause the tetragonal phase to convert to monoclinic, with the associated volume expansion. This phase transformation can then put the crack into compression, retarding its growth, and enhancing the fracture toughness. This mechanism is known as transformation toughening, and significantly extends the reliability and lifetime of products made with stabilized zirconia.
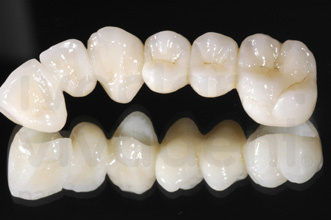 Zirconia - ceramic materials for dental-lab applications.
Zirconia - ceramic materials for dental-lab applications.
A special case of zirconia is that of tetragonal zirconia polycrystaline or TZP, which is indicative of polycrystalline zirconia composed of only the metastable tetragonal phase.The cubic phase of zirconia also has a very low thermal conductivity, which has led to its use as a thermal barrier coating or TBC in jet and diesel engines to allow operation at higher temperatures. Thermodynamically the higher the operation temperature of an engine, the greater the possible efficiency (see Carnot heat engine). As of 2004, a great deal of research is ongoing to improve the quality and durability of these coatings. It is used as a refractory material, in insulation, abrasives, enamels and ceramic glazes. Stabilized zirconia is used in oxygen sensors and fuel cell membranes because it has the ability to allow oxygen ions to move freely through the crystal structure at high temperatures. This high ionic conductivity (and a low electronic conductivity) makes it one of the most useful electroceramics.
 Multi colour Cubic zirconia
Multi colour Cubic zirconia
The ZrO2 band gap is dependent on the phase (cubic, tetragonal, monoclinic, or amorphous) and preparation methods, with typical estimates from 5-7 eV. This material is also used in the manufacture of subframes for the construction of dental restorations such as crowns and bridges, which are then veneered with a conventional feldspathic porcelain. Zirconium dioxide can occur as a white powder which possesses both acidic and basic properties. On account of its infusibility and brilliant luminosity when incandescent, it was used as an ingredient of sticks for limelight. Zirconia is also an important high-k dielectric material that is being investigated for potential applications as an insulator in transistors in future nanoelectronic devices.
 An eloctromagnetically levitated ball of molten titanium-zirconium-nickel alloy. As the floating liquid ball cools and solidifies, it reveals information about why liquids resist turning into solids.
An eloctromagnetically levitated ball of molten titanium-zirconium-nickel alloy. As the floating liquid ball cools and solidifies, it reveals information about why liquids resist turning into solids.
Cubic zirconia (or CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard, optically flawless and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called “cubic zirconium”.
 Cubic Zirconia
Cubic Zirconia
Because of its low cost, durability, and close visual likeness to diamond, synthetic cubic zirconia has remained the most gemologically and economically important competitor for diamonds since 1976. Its main competitor as a synthetic gemstone is the more recently cultivated material, synthetic moissanite.
 Zirconium Ball
Zirconium Ball
Cubic zirconia is a synthetic gemstone that very closely resembles diamonds. Because of its startling diamond-like appearance and inexpensive price tag, cubic zirconia is a highly popular gemstone used most frequently in jewelry such as rings, earrings, bracelets and pendants. Although cubic zirconia is synthetic, it is inspired by its natural counterpart, zirconium oxide (ZrO2), first discovered in 1892 but too rare to be commercially profitable. Through a series of separate experiments by German and Soviet scientists, zirconium oxide and yttrium oxide were eventually melted together at temperatures reaching 4,982ºF (2,750ºC) to grow cubic zirconia crystals in the laboratory.
 Zirconium floating ball valve
Zirconium floating ball valve
Cubic zirconia is crystalline, flawless, and clear enough to rate a “D” on the diamond scale for color. Though usually colorless, it can also be made in nearly any color, including soft yellow, characteristic of some diamonds. Cubic zirconia sparkles brighter than crystal and is harder than most gems, making it very durable.
It also weighs about 65% more than diamond. However, if there is an obvious difference between the two to the untrained eye, it is that cubic zirconia has a higher dispersion rate than diamond.
You might also like
| Advanced Ceramics A ceramic is an inorganic, nonmetallic... | How is Titanium made? Titanium is known as a transition... | Titanium and It’s Alloys Titanium Metal Titanium was... | Cast Iron Cast iron is derived from pig iron,... |





 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope