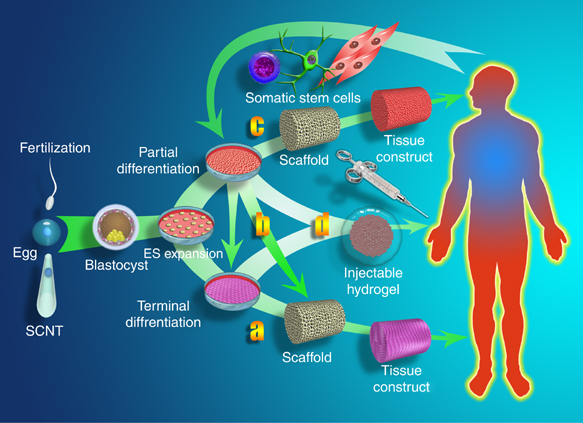
 Multiple roles for biomaterials in stem cell TE, source:https://www.nature.com/
Multiple roles for biomaterials in stem cell TE, source:https://www.nature.com/
A biomaterial is any material, natural or man-made, that comprises whole or part of a living structure or biomedical device which performs, auguments, or replaces a natural function. Biomaterials must be compatible with the body, and there are often issues of biocompatibility which must be resolved before a product can be placed on the market and used in a clinical setting.
Because of this, biomaterials are usually subjected to the same requirements of those undergone by new drug therapies. All manufacturing companies are also required to ensure traceability of all of their products so that if a defective product is discovered, others in the same batch may be traced.
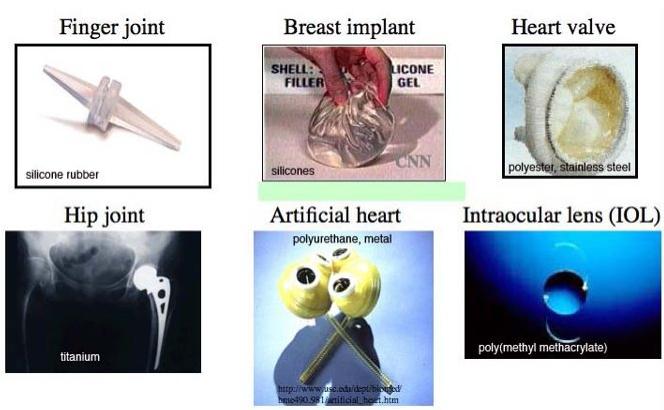
Applications of Biomaterials
A biomaterial is any matter, surface, or construct that interacts with biological systems. The development of biomaterials, as a science, is about fifty years old. The study of biomaterials is called biomaterials science. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.

The iridescent nacre inside a nautilus shell.
Biomaterials are materials (synthetic and natural; solid and sometimes liquid) that are used in medical devices or in contact with biological systems. There is also a powerful human side to biomaterials that considers ethics, law and the health care delivery system. This brief introduction overviews some key characteristics of the field of biomaterials and outlines issues and major subdivisons.
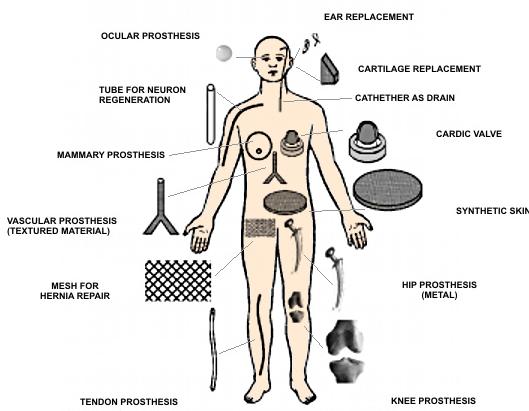 Biomaterials at the human body
Biomaterials at the human body
Although biomaterials are primarily used for medical applications, they are also used to grow cells in culture, to assay for blood proteins in the clinical laboratory, in processing biomolecules in biotechnology, for fertility regulation implants in cattle, in diagnostic gene arrays, in the aquaculture of oysters and for investigational cell-silicon “biochips.” The commonality of these applications is the interaction between biological systems and synthetic or modified natural materials.
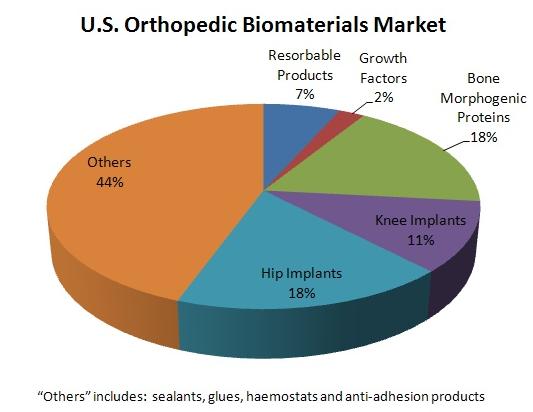 Biomaterials Market, source: MedMarket Diligence
Biomaterials Market, source: MedMarket Diligence
Biomaterials can generally be produced either in nature or synthesized in the laboratory using a variety of chemical approaches utilizing metallic components or ceramics. They are often used and/or adapted for a medical application, and thus comprises whole or part of a living structure or biomedical device which performs, augments, or replaces a natural function. Such functions may be benign, like being used for a heart valve, or may be bioactive with a more interactive functionality such as hydroxy-apatite coated hip implants.
What is Subject of Biomaterials ?
Toxicology
A biomaterial should not be toxic, unless it is specifically engineered for such requirements (for example, a “smart bomb” drug delivery system that targets cancer cells and destroys them). Since the nontoxic requirement is the norm, toxicology for biomaterials has evolved into a sophisticated science. It deals with the substances that migrate out of biomaterials. For example, for polymers, many low-molecular-weight “leachables” exhibit some level of physiologic activity and cell toxicity. It is reasonable to say that a biomaterial should not give off anything from its mass unless it is specifically designed to do so. Toxicology also deals with methods to evaluate how well this design criterion is met when a new biomaterial is under development.
Biocompatibility
The understanding and measurement of biocompatibility is unique to biomaterials science. Unfortunately, we do not have precise definitions or accurate measurements of biocompatibility. More often than not, biocompatibility is defined in terms of performance or success at a specific task. Thus, for a patient who is doing well with an implanted Dacron fabric vascular prosthesis, few would argue that this prosthesis is not “biocompatible.
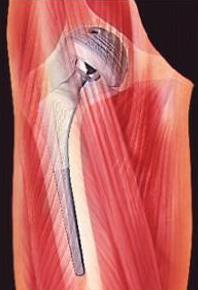 The image shows a implanted artificial hip joint.
The image shows a implanted artificial hip joint.
However, the prosthesis probably did not re-cellularize (though it was designed to do so) and also can throw off blood clots (emboli), though the emboli in this case usually have little clinical consequence. This operational definition of biocompatible (“the patient is alive so it must be biocompatible”) offers us little insight in designing new or improved vascular prostheses. It is probable that biocompatibility may one day be defined for applications in soft tissue, hard tissue, and the cardiovascular system (blood compatibility).
Functional Tissue Structure and Pathobiology
Biomaterials incorporated into medical devices are implanted into tissues and organs. Therefore, the key principles governing the structure of normal and abnormal cells, tissues and organs, the techniques by which the structure and function of normal and abnormal tissue are studied, and the fundamental mechanisms of disease processes are critical considerations to workers in the field.
Healing
Special processes are invoked when a material or device heals in the body. Injury to tissue will stimulate the well-defined inflammatory reaction sequence that leads to healing. Where a foreign body (e.g., an implant) is present in the wound site (surgical incision), the reaction sequence is referred to as the “foreign body reaction.” The normal response of the body will be modulated because of the solid implant. Furthermore, this reaction will differ in intensity and duration depending upon the anatomical site involved. An understanding of how a foreign object alters the normal inflammatory reaction sequence is an important concern for the biomaterials scientist.
Dependence on Specific Anatomical Sites of Implantation
Consideration of the anatomical site of an implant is essential. An intraocular lens may go into the lens capsule or the anterior chamber of the eye. A hip joint will be implanted in bone across an articulating joint space. A heart valve will be sutured into cardiac muscle and will contact both soft tissue and blood. A catheter may be placed in an artery, a vein or the urinary tract. Each of these sites challenges the biomedical device designer with special requirements for geometry, size, mechanical properties, and bioresponses.
Mechanical and Performance Requirements
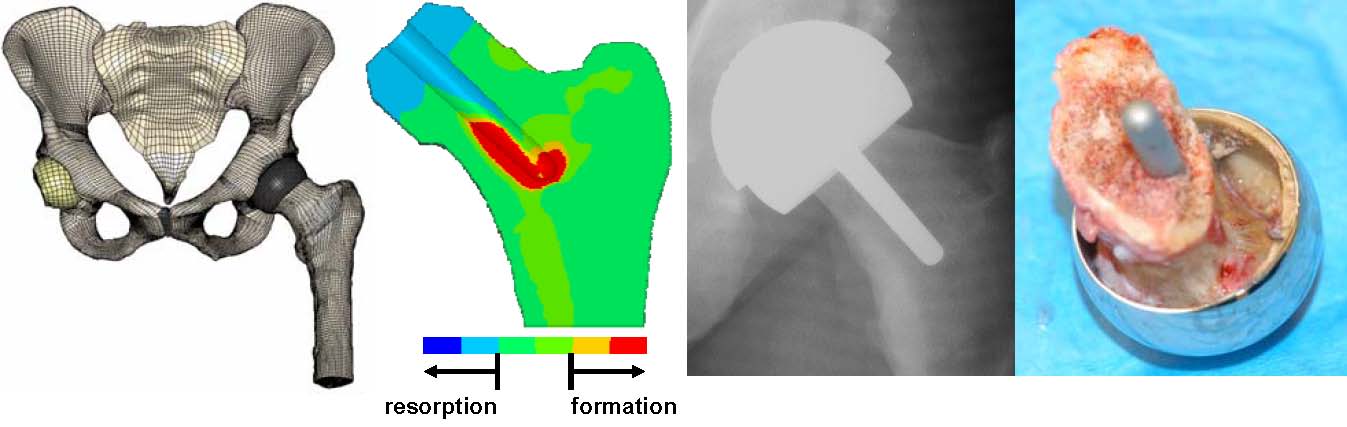
Biomaterials and devices have mechanical and performance requirements that originate from the physical (bulk) properties of the material. There are three categories of such requirements: mechanical performance, mechanical durability and physical properties. First, consider mechanical performance. A hip prosthesis must be strong and rigid. A tendon material must be strong and flexible. A heart valve leaflet must be flexible and tough. A dialysis membrane must be strong and flexible, but not elastomeric. An articular cartilage substitute must be soft and elastomeric.
Orthopaedic Implants
Then, we must address mechanical durability. A catheter may only have to perform for 3 days. A bone plate may fulfill its function in 6 months or longer. A leaflet in a heart valve must flex 60 times per minute without tearing for the lifetime of the patient (realistically, at least for 10 or more years). A hip joint must not fail under heavy loads for more than 10 years. The bulk physical properties will also address other aspects of performance. The dialysis membrane has a specified permeability, the articular cup of the hip joint must have high lubricity, and the intraocular lens has clarity and refraction requirements. To meet these requirements, design principles from physics, chemistry, mechanical engineering, chemical engineering, and materials science are invoked.
Industrial Involvement
A significant basic research effort is now under way to understand how biomaterials function and how to optimize them. At the same time, companies are producing implants for use in humans and, appropriate to the mission of a company, earning profits on the sale of medical devices. Thus, although we are now only learning about the fundamentals of biointeraction, we manufacture and implant millions of devices in humans. How is this dichotomy explained? Basically, as a result of considerable experience we now have a set of materials that performs satisfactorily in the body. The medical practitioner can use them with reasonable confidence, and the performance in the patient is largely acceptable. Though the devices and materials are far from perfect, the complications associated with the devices are less than the complications of the original diseases.
 Small intestinal submucosa (SIS) is a biomaterial that can induce host tissue proliferation and regeneration when it is implanted at various tissue sites.
Small intestinal submucosa (SIS) is a biomaterial that can induce host tissue proliferation and regeneration when it is implanted at various tissue sites.
The complex balance between the desire to alleviate suffering and death, the excitement of new scientific ideas, the corporate imperative to turn a profit, the risk/benefit relationship and the mandate of the regulatory agencies to protect the public forces us to consider the needs of many constituencies. Obviously, ethical concerns enter into the picture. Also, companies have large investments in the manufacture, quality control, clinical testing, regulatory clearance, and distribution of medical devices. How much of an advantage (for the company and the patient) will be realized in introducing an improved device? The improved device may indeed work better for the patient.
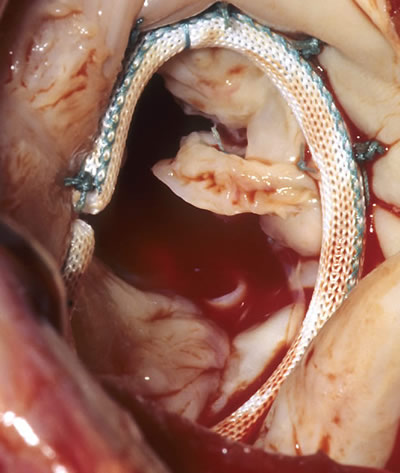 The Annuloplasty ring is used to retain the diameter of the opening of the heart valve.
The Annuloplasty ring is used to retain the diameter of the opening of the heart valve.
However, the company will incur a large expense that will be perceived by the stockholders as reduced profits. Moreover, product liability issues are a major concern of manufacturers. The industrial side of the biomaterials field raises questions about the ethics of withholding improved devices from people who need them, the market share advantages of having a better product, and the gargantuan costs (possibly non-recoverable) of introducing a new product into the medical marketplace. If companies did not have the profit incentive, would there be any medical devices, let alone improved ones, available for clinical application?
Ethics
A wide range of ethical considerations impact biomaterials science. Like most ethical questions, an absolute answer may be difficult to come by. Some articles have addressed ethical questions in biomaterials and debated the important points (Saha and Saha, 1987; Schiedermayer and Shapiro, 1989).
Regulation
The consumer (the patient) demands safe medical devices. To prevent inadequately tested devices and materials from coming on the market, and to screen out individuals clearly unqualified to produce biomaterials, the United States government has evolved a complex regulatory system administered by the US Food and Drug Administration (FDA). Most nations of the world have similar medical device regulatory bodies. The International Standards Organization (ISO) has introduced international standards for the world community. Obviously, a substantial base of biomaterials knowledge went into establishing these standards. The costs to comply with the standards and to implement materials, biological, and clinical testing are enormous. Introducing a new biomedical device to the market requires a regulatory investment of tens of millions of dollars.
The numbers of medical devices used each year in humans is very large.
Biomaterials researchers and clinicians using biomaterials will have an appreciation of materials science and chemistry. Many different synthetic and modified natural materials are used in biomaterials and some understanding of the differing properties of these materials is important.
 Heart Valve
Heart Valve
A heart valve may be fabricated from polymers, metals, and carbons. A hip joint might be fabricated from metals and polymers (and sometimes ceramics) and will be interfaced to the body via a polymeric bone cement. In these examples, a single device uses many different materials, each with special properties and biological interactions.
You might also like
Random Posts
- Metallurgy Glossary
Activity: A function of the chemical potential of a system.
Alloy: A metallic substance that is composed of two or more... - The Casting Process Pictures
These are the metallurgy pictures jobs and activities in the Metal Casting. There are very hot but interesting... - Shape Memory Alloy
A shape memory alloy (SMA, smart metal, memory metal, memory alloy, muscle wire, smart alloy) is an alloy that "remember... - Forging
A forge is a hearth used for forging. The term "forge" can also refer to the workplace of a smith or a blacksmith, altho... - Bainite
Bainite is an acicular microstructure (not a phase) that forms in steels at temperatures from approximately 250-550°C (d...
 Multiple roles for biomaterials in stem cell TE, source:https://www.nature.com/
Multiple roles for biomaterials in stem cell TE, source:https://www.nature.com/ Biomaterials at the human body
Biomaterials at the human body Biomaterials Market, source: MedMarket Diligence
Biomaterials Market, source: MedMarket Diligence The image shows a implanted artificial hip joint.
The image shows a implanted artificial hip joint. Small intestinal submucosa (SIS) is a biomaterial that can induce host tissue proliferation and regeneration when it is implanted at various tissue sites.
Small intestinal submucosa (SIS) is a biomaterial that can induce host tissue proliferation and regeneration when it is implanted at various tissue sites. The Annuloplasty ring is used to retain the diameter of the opening of the heart valve.
The Annuloplasty ring is used to retain the diameter of the opening of the heart valve. Heart Valve
Heart Valve




 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope