Galvanized Steel - a Definition
Sheet, strip, or other steel item coated with a thin layer of zinc for corrosion resistance. Thickness of the zinc layer determines the length of resistance period. Galvanized steel has gone through a chemical process to keep it from corroding. The steel gets coated in layers of zinc because rust won’t attack this protective metal. For countless outdoor, marine, or industrial applications, galvanized steel is an essential fabrication component.
 Galvanized steel has gone through a chemical process to keep it from corroding
Galvanized steel has gone through a chemical process to keep it from corroding
Zinc coatings prevent corrosion of the protected metal by forming a physical barrier, and by acting as a sacrificial anode if this barrier is damaged. When exposed to the atmosphere, zinc reacts with oxygen to form zinc oxide, which further reacts with water molecules in the air to form zinc hydroxide. Finally zinc hydroxide reacts with carbon dioxide in the atmosphere to yield a thin, impermeable, tenacious and quite insoluble dull gray layer of zinc carbonate which adheres extremely well to the underlying zinc, so protecting it from further corrosion, in a way similar to the protection afforded to aluminium and stainless steels by their oxide layers.
Hot-dip galvanizing deposits a thick robust layer that may be more than is necessary for the protection of the underlying metal in some applications. Here, a thinner form of galvanizing is applied by electroplating, called “electrogalvanization”. The hot-dip process slightly reduces the strength of the base metal, which is a consideration for the manufacture of wire rope and other highly-stressed products. Some nails made today are electro-galvanized.
Thin coatings cannot remain intact indefinitely when subject to surface abrasion, and the galvanic protection offered by zinc can be sharply contrasted to more noble metals. The size of crystallites in galvanized coatings is an aesthetic feature, known as spangle. Visible crystallites are rare in other engineering materials. Protective coatings for steel constitute the largest use of zinc and rely upon the galvanic or sacrificial property of zinc relative to steel.
Thermal diffusion galvanizing, a form of Sherardizing, provides a zinc coating on iron or copper based materials partially similar to hot dip galvanizing. The final surface is different than hot-dip Galvanizing, all of its zinc is alloyed. Zinc is applied in a powder form with “accelerator chemicals” (generally sand, but other chemicals are patented). The parts and the zinc powder are tumbled in a sealed drum while it is heated to slightly below zinc’s melting temperature. Due to the chemicals added to the zinc powder, the zinc/iron makes an alloy at a lower temperature than hot dip galvanizing. The dull-grey crystal structure formed by the process bonds stronger with paint, powder coating, and rubber overmolding processes than other methods. It is a preferred method for coating small, complex-shaped metals and smoothing in rough surfaces on items formed with powder metal.
Although galvanizing will inhibit attack of the underlying steel, rusting will be inevitable, especially due to natural acidity of rain. For example, corrugated iron sheet roofing will start to degrade within a few years despite the protective action of the zinc coating. Marine and salty environments also lower the lifetime of galvanized iron because the high electrical conductivity of sea water increases the rate of corrosion. Galvanized car frames exemplify this they corrode much quicker in cold environments due to road salt. Galvanized steel can last for many years if other means are maintained, such as paint coatings and additional sacrificial anodes.
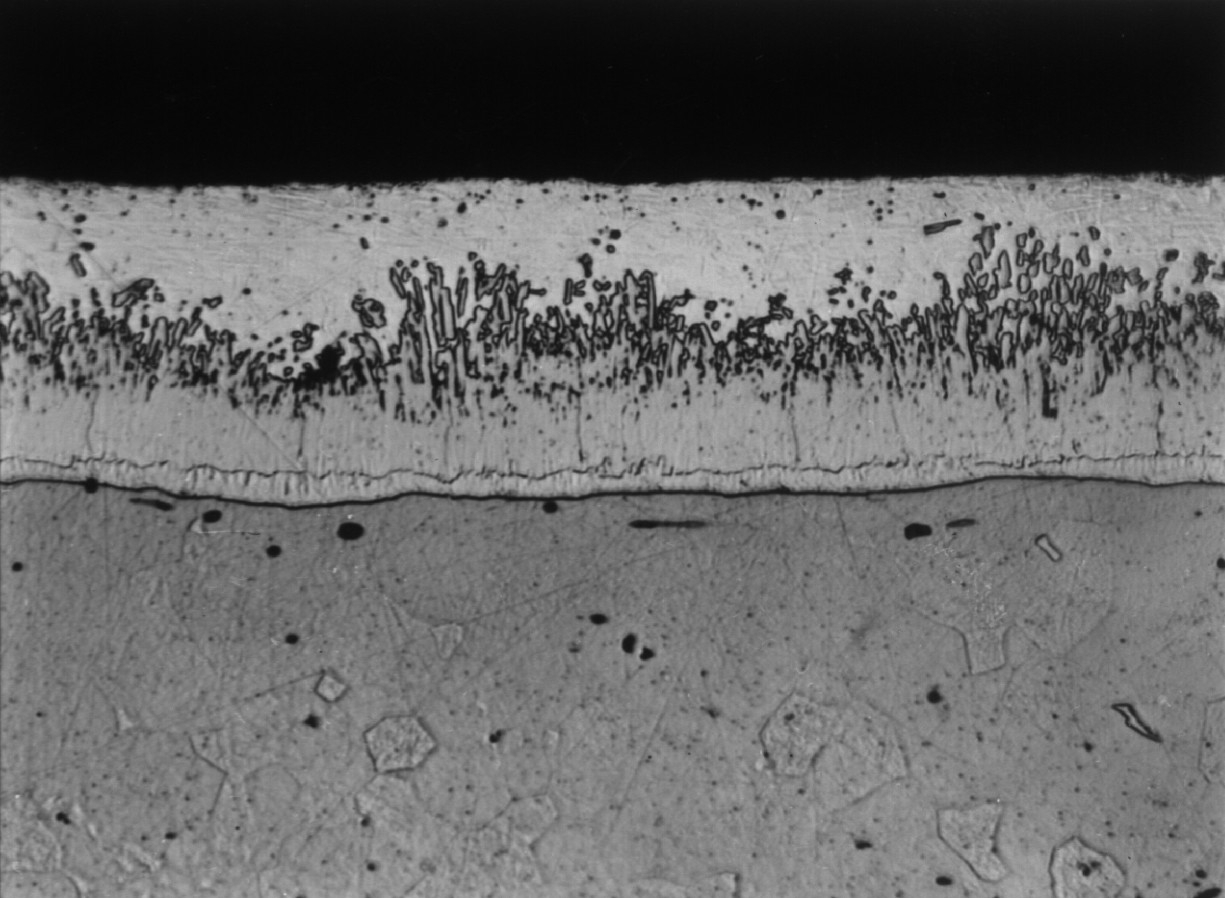 Microstructure of Galvanized Steel
Microstructure of Galvanized Steel
The principal method of making steel resist corrosion is by alloying it with another metal, zinc. When steel is submerged in melted zinc, the chemical reaction permanently bonds the zinc to the steel through galvanizing. Therefore, the zinc isn’t exactly a sealer, like paint, because it doesn’t just coat the steel, it actually permanently becomes a part of it. This remains a useful and broadly applied technology, but the term “galvanization” has largely come to be associated with zinc coatings, to the exclusion of other metals.Galvanic paint, a precursor to hot-dip galvanization, was patented by Stanislas Sorel, of Paris, France in December, 1837.
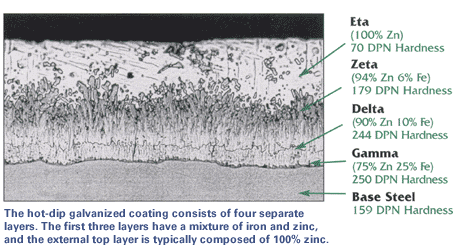
The zinc goes through a reaction with the iron molecules within the steel to form galvanized steel. The most external layer is all zinc, but successive layers are a mixture of zinc and iron, with an interior of pure steel. These multiple layers are responsible for the amazing property of the metal to withstand corrosion-inducing circumstances, such as saltwater or moisture.
Zinc also protects the steel by acting as a “sacrificial layer.” If, for some reason, rust does take hold on the surface of galvanized steel, the zinc will get corroded first. This allows the zinc that is spread over the breach or scratch to prevent rust from reaching the steel.Steel often gets galvanized after individual parts have been formed, such as braces, nails, screws, beams, or studs. However, raw galvanized steel in sheets will withstand some bending and forming without flaking.
Galvanized steel can be found almost everywhere. You might be living in a steel frame house. Many people work in an office with metal roofing made of galvanized steel. In current use, the term refers to the coating of steel or iron with zinc. The value of galvanizing stems from the relative corrosion resistance of zinc, which, under most service conditions, is considerably less than those of iron and steel. The zinc therefore serves as a sacrificial anode, so that it cathodically protects exposed steel. This means that even if the coating is scratched or abraded, the exposed steel will still be protected from corrosion by the remaining zinc - an advantage absent from paint, enamel, powder coating and other methods. Galvanizing is also favored as a means of protective coating because of its low cost, ease of application and comparatively long maintenance-free service life.
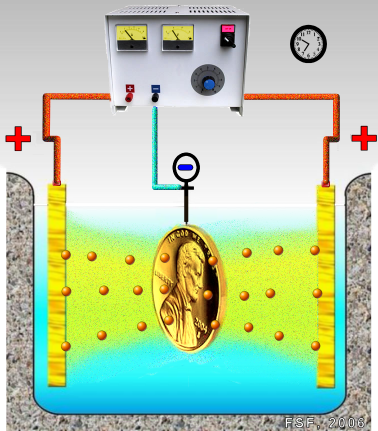
The term galvanizing, while technically referring specifically to the application of zinc coating by the use of a galvanic cell (also known as electroplating), is also generally understood to include hot-dip zinc coating. The practical difference is that hot-dip galvanization produces a thick, durable and matte gray coating - electroplated coatings tend to be thin and brightly reflective. Due to its thinness, the zinc of electroplated coatings is quickly depleted, making them unsuitable for outdoor applications (except in very dry climates). When combined with subsequent painting (which slows zinc consumption), electroplating is durable enough to be used in some premium auto body coatings.
Nonetheless, electroplating is used on its own for many outdoor applications because it is cheaper than hot dip zinc coating and looks good when new. Another reason not to use hot dip zinc coating is that for bolts and nuts size M10 or smaller, the thick hot-dipped coating uses up too much of the threads, which reduces strength (because the dimension of the steel prior to coating must be reduced for the fasteners to fit together). This means that for cars, bicycles and many other ‘light’ mechanical products, the alternative to electroplating bolts and nuts is not hot dip zinc coating but making the bolts and nuts from stainless steel (known by the corrosion grades A4 and A2).
Electroplated steel is visually indistinguishable from stainless steel when new. To determine whether a part is electroplated or stainless steel, apply a magnet. Hot dip galvanizing is the process of applying a zinc coating to fabricated iron or steel material by immersing the material in a bath consisting primarily of molten zinc. The simplicity of the galvanizing process is a distinct advantage over other methods of providing corrosion protection. Galvanizing forms a metallurgical bond between the zinc and the underlying steel or iron, creating a barrier that is part of the metal itself.
The process of hot-dip galvanizing results in a metallurgical bond between zinc and steel with a series of distinct iron-zinc alloys. The resulting coated steel can be used in much the same way as uncoated. Galvanized steel can be welded; however, one must exercise caution around the resulting zinc fumes. Galvanized steel is suitable for high-temperature applications of up to 392°F (200°C). The use of galvanized steel at temperatures above this will result in peeling of the zinc at the intermetallic layer. Electrogalvanized sheet steel is often used in automotive manufacturing to enhance the corrosion performance of exterior body panels, this is however a completely different process.
Lead is often added to the molten zinc bath to improve the fluidity of the bath (thus limiting excess zinc on the dipped product by improved drainage properties), helps prevent floating dross, makes dross recycling easier and protects the kettle from uneven heat distribution from the burners. Lead is either added to primary Z1 Grade Zinc or already contained in used secondary zinc. A third, declining method is to use low Z5 Grade Zinc.
Steel strip can be hot-dip galvanized in a continuous line. Hot-dip galvanized steel strip (also sometimes loosely referred to as galvanized iron) is extensively used for applications requiring the strength of steel combined with the resistance to corrosion of zinc. Applications include: roofing and walling, safety barriers, handrails, consumer appliances and automotive body parts. Galvanised steel is also used in most heating and cooling duct systems in buildings.Individual metal articles, such as steel girders or wrought iron gates, can be hot-dip galvanized by a process called batch galvanizing. Other modern techniques have largely replaced hot-dip for these sorts of roles.









 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope