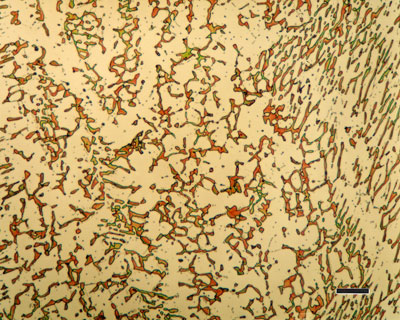
How can stainless steel corrode?
All “stainless” steels can corrode under the wrong conditions. Remember that most of the metal is still iron and can corrode or rust depending on how much stress it is exposed to. One of the worst things you can do to stainless steel is expose it to chlorides. The better grades can withstand a lot of abuse, but most grades can eventually fail when exposed to chlorides in bad atmospheres. Some grades of “stainless” steel have very poor resistance to corrosion. They are marginally better than carbon steel, but when correctly hardened and passivated or electropolished can give fairly good performance. The grade of steel must reflect the exposure conditions and performance required.
 Corrosion Stainless Steel Pipe
Corrosion Stainless Steel Pipe
The main reason for the existence of the stainless steels is their resistance to corrosion. Chromium is the main alloying element, and the steel should contain at least 11 %. Chromium is a reactive element, but it and its alloys passivate and exhibit excellent resistance to many environments. Higher Cr contents may be necessary for to improve the corrosion resistance of stainless steels in more aggressive media. Among other alloying elements, Nickel is the most important : it is added to control the alloy microstructure and to improve the corrosion resistance in acidic or caustic media. The addition of other elements such as Molybdenum, Tungsten, Copper, Silicon, Titanium, Niobium, Nitrogen enables a wide range of properties to be obtained.
You might also like
| What is Stainless Steel? Stainless Steel - A Definition Stainless... | Austenite - Gamma Iron Austenite - a Definition Austenite also... | Microstructure of Metals Microstructure of Metals Microstructure... | Galvanized Steel - a Definition What is Galvanized Steel ? Sheet, strip,... |


 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope