Carburizing
What is Carburizing ?
Carburizing is a process of adding Carbon to the surface. This is done by exposing the part to a Carbon rich atmosphere at an elevated temperature and allows diffusion to transfer the Carbon atoms into steel. This diffusion will work only if the steel has low carbon content, because diffusion works on the differential of concentration principle. If, for example the steel had high carbon content to begin with, and is heated in a carbon free furnace, such as air, the carbon will tend to diffuse out of the steel resulting in Decarburization.
Carburizing Processing
Carburizing is the addition of carbon to the surface of low-carbon steels at temperatures generally between 850 and 950°C (1560 and 1740°F), at which austenite, with its high solubility for carbon, is the stable crystal structure. Hardening is accomplished when the high-carbon surface layer is quenched to form martensite so that a high-carbon martensitic case with good wear and fatigue resistance is superimposed on a tough, low-carbon steel core. Carburizing steels for case hardening usually have base-carbon contents of about 0.2%, with the carbon content of the carburized layer generally being controlled at between 0.8 and 1% C. However, surface carbon is often limited to 0.9% because too high a carbon content can result in retained austenite and brittle martensite.
A method that overcomes both of these major problems, yet retains the desirable features of a simple atmosphere and permissible operating temperature is plasma or ion carburizing.
To summarize, carburizing methods include :
- Gas carburizing
- Vacuum carburizing
- Plasma carburizing
- Salt bath carburizing
- Pack carburizing
These methods introduce carbon by the use of gas (atmospheric-gas, plasma, and vacuum carburizing), liquids (salt bath carburizing), or solid compounds (pack carburizing). All of these methods have limitations and advantages, but gas carburizing is used most often for large-scale production because it can be accurately controlled and involves a minimum of special handling.
Vacuum carbunzing and plasma carburizing have found applications because of the absence of oxygen in the furnace atmosphere. Salt bath and pack carburizing arc still done occasionally, but have little commercial importance today.
Carburizing is the addition of carbon to the surface of low-carbon steels at temperatures generally between 850 and 950°C (1560 and 1740°F), at which austenite, with its high solubility for carbon, is the stable crystal structure. Hardening is accomplished when the high-carbon surface layer is quenched to form martensite so that a high-carbon martensitic case with good wear and fatigue resistance is superimposed on a tough, low-carbon steel core.
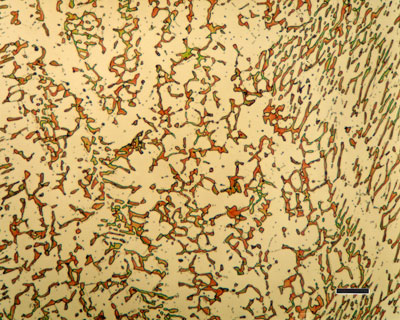
The Carbon content in the steel determines whether it can be directly hardened. If the Carbon content is low (less than 0.25% for example) then an alternate means exists to increase the Carbon content of the surface. Depending on the amount of time and temperature, the affected area can vary in carbon content. Longer carburizing times and higher temperatures lead to greater carbon diffusion into the part as well as increased depth of carbon diffusion. When the iron or steel is cooled rapidly by quenching, the higher carbon content on the outer surface becomes hard via the transformation from austenite to martensite, while the core remains soft and tough as a ferritic and/or pearlite microstructure.This manufacturing process can be characterized by the following key points: It is applied to low-carbon workpieces; workpieces are in contact with a high-carbon gas, liquid or solid, it produces a hard workpiece surface; workpiece cores largely retain their toughness and ductility and it produces case hardness depths of up to 0.25 inches (6.4 mm).
Carburization of steel involves a heat treatment of the metallic surface using a source of carbon. Early carburization used a direct application of charcoal packed onto the metal (initially referred to as case hardening), but modern techniques apply carbon-bearing gases or plasmas (such as carbon dioxide or methane). Carburizing is the addition of carbon to the surface of low-carbon steels at temperatures generally between 850 and 950°C (1560 and 1740°F), at which austenite, with its high solubility for carbon, is the stable crystal structure. Hardening is accomplished when the high-carbon surface layer is quenched to form martensite so that a high-carbon martensitic case with good wear and fatigue resistance is superimposed on a tough, low-carbon steel core.
In its earliest application, parts were simply placed in a suitable container and covered with a thick layer of carbon powder (pack carburizing). In gas carburizing, the parts are surrounded by a carbon-bearing atmosphere that can be continuously replenished so that a high carbon potential can be maintained. In efforts required to simplify the atmosphere, carburizing in an oxygen-free environment at very low pressure (vacuum carburizing) has been explored and developed into a viable and important alternative.
Furthermore, because the parts are heated in an oxygen-free environment, the carburizing temperature may be increased substantially without the risk of surface or grain-boundary oxidation. Because vacuum carburizing is conducted at very low pressures, and the rate of flow of the carburizing gas into the furnace is very low, the carbon potential of the gas in deep recesses and blind holes is quickly depleted. Unless this gas is replenished, a great nonuniformity in case depth over the surface of the part is likely to occur. If, in an effort to overcome this problem, the gas pressure is increased significantly, another problem arises, that of free-carbon formation, or sooting. Case hardness of carburized steels is primarily a function of carbon content. When the carbon content of the steel exceeds about 0.50% additional carbon has no effect on hardness but does enhance hardenability. Carbon in excess of 0.50% may not be dissolved, which would thus require temperatures high enough to ensure carbon-austenite solid solution.
Case depth of carburized steel is a function of carburizing time and the available carbon potential at the surface. When prolonged carburizing times are used for deep case depths, a high carbon potential produces a high surface-carbon content, which may thus result in excessive retained austenite or free carbides. Consequently, a high carbon potential may be suitable for short carburizing times but not for prolonged carburizing.
Gas carburizing is normally carried out at a temperature within the range of 900 to 950 °C. In oxy-acetylene welding, a carburizing flame is one with little oxygen, which produces a sooty, lower-temperature flame. Carburizing steels for case hardening usually have base-carbon contents of about 0.2%, with the carbon content of the carburized layer generally being controlled at between 0.8 and 1% C. However, surface carbon is often limited to 0.9% because too high a carbon content can result in retained austenite and brittle martensite.
Pack carburizing containers are usually made of carbon steel coated with aluminum or heat-resisting nickle-chromium alloy and sealed at all openings with fire clay. There are different types of elements or materials that can be used to perform this process, but these mainly consist of high carbon content material.
A few typical hardening agents include carbon monoxide gas (CO), sodium cyanide and barium chloride, or hardwood charcoal. In gas carburizing, the CO is given off by propane or natural gas. In pack carburizing, carbon monoxide is given off by coke or hardwood charcoal.
Plasma carburization is increasingly used in major industrial regimes to improve the surface characteristics (such as wear and corrosion resistance, hardness and load-bearing capacity, in addition to quality-based variables) of various metals, notably stainless steels. The process is used as it is environmentally friendly (in comparison to gaseous or solid carburizing). It also provides an even treatment of components with complex geometry (the plasma can penetrate into holes and tight gaps), making it very flexible in terms of component treatment.
Steels made to coarse grain practices can be carburized if a double quench provides grain refinement. Many alloy steels for case hardening are now specified on the basis of core hardenability. First, in a case-hardened steel, the hardenability of both case and core must be considered.
The relationship between the thermal gradient and the carbon gradient during quenching of a carburized part can make a measurable difference in the case depth as measured by hardness. That is, an increase in base hardenability can produce a higher proportion of martensite for a given carbon level, yielding an increased measured case depth. Therefore, a shallower carbon profile and shorter carburizing time could be used to attain the desired result in a properly chosen steel.









 Alloy Suppliers
Alloy Suppliers  Aluminum
Aluminum  Aluminum Extrusions
Aluminum Extrusions  Copper-Brass-Bronze
Copper-Brass-Bronze  Nickel
Nickel  Magnets
Magnets  Stainless Steel
Stainless Steel  Stainless Steel Tubing
Stainless Steel Tubing  Steel Service Centers
Steel Service Centers  Titanium
Titanium  Tungsten
Tungsten  Wire Rope
Wire Rope