Nature of Metals
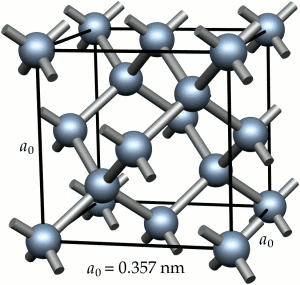
Metallic bonding is a consequence of the metal atoms giving up valence electrons to a ‘free electron gas‘. Metallic structures at the atomic level are then envisaged as almost close-packed arrays of metal ions surrounded by the electron gas. The bonding is, in most cases, non-directional. As a consequence the common metallic crystal structures are face-centred cubic, e.g. Cu, Al, Ni, or body-centred cubic, e.g. Fe. (Some metals exist with a hexagonal close-packed structure, e.g. Zn, Cd, but these are not commonly used for structural applications.)
Metals (and alloys) with cubic structures exhibit four characteristic metallic properties, namely:
- good ductility (or malleability).
- high thermal conductivity.
- high electrical conductivity.
- metallic lustre.
Ductility is a consequence of the lack of directionality in the bonding of the atoms and the close-packed nature of the crystal structures which normally allows profuse crystallographic slip to occur under stress. The non-directionality in the bonding also allows thermal vibrations to be readily transmitted from one vibrating atom to its neighbours, hence the high thermal conductivity. The existence of free electrons provides for high electrical conductivity. These free electrons are also responsible for metallic lustre since incident light of a wide range of wavelengths can be readily absorbed and re-radiated.
You might also like
| Types of Materials Metals: Metals are elements... | Semiconductor Materials A semiconductor is a substance,... | Structure and Components of Steel The engineering properties of steel,... | Corrosion Corrosion is the disintegration of an engineered... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope