Hydrogen Embrittlement
Hydrogen embrittlement reduces ductility, often to the point where metals behave like ceramics. Hydrogen embrittlement failures have even been observed in unassembled parts in inventory, a phenomenon known as “shelf popping”.
Sudden brittle fracture in high strength steels resulting from hydrogen embrittlement represents an extremely dangerous phenomenon to industry, particularly since it is usually the result of factors that occur during the manufacturing process.
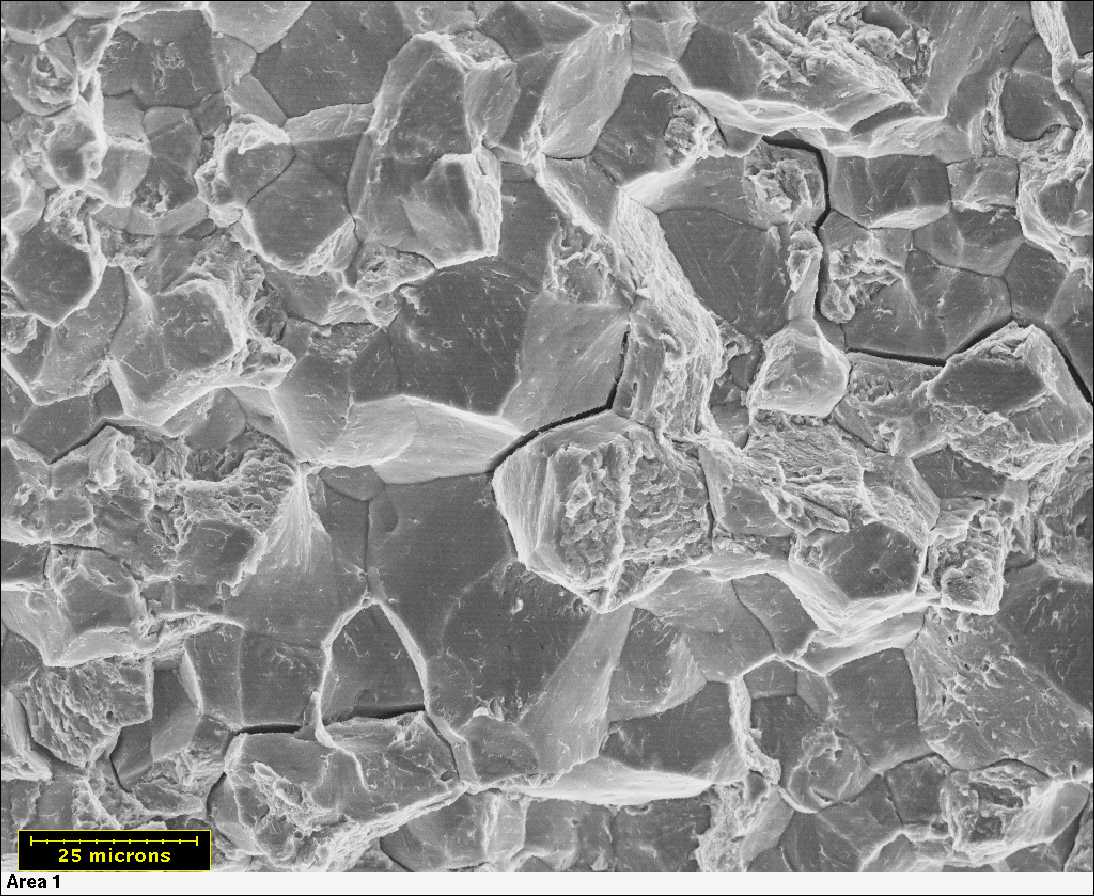
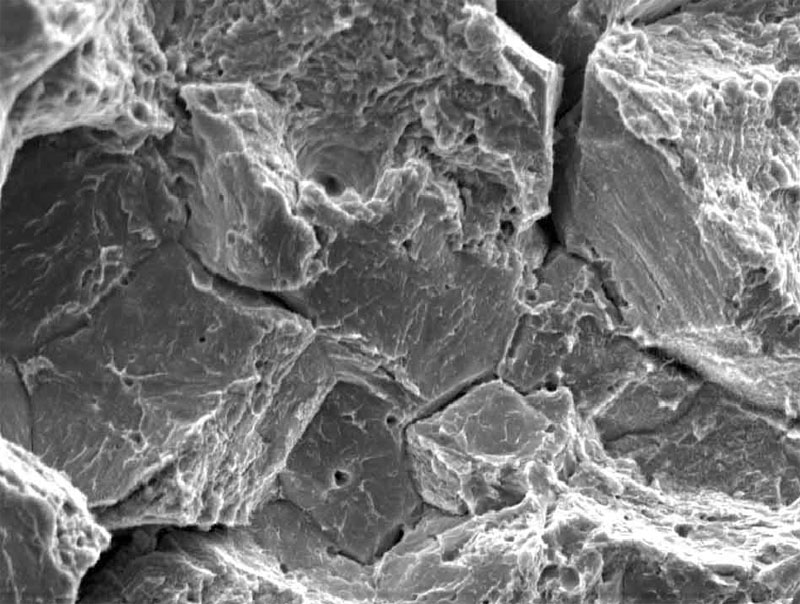 Hydrogen embrittlement cracking is primarily intergrannular
Hydrogen embrittlement cracking is primarily intergrannular
Generally, the higher the strength of the steel, the more at risk it is to hydrogen embrittlement and the more vulnerable it is to lower levels of hydrogen. Although hydrogen embrittlement occurs in many different metal alloys, high strength steel appears to be the most sensitive, is the most widely used and accounts for the largest number of hydrogen embrittlement failures. In response to demands for more strength, “radical” heat treatments to tensile strengths approaching 200,000 psi were applied to 4130 and other “anemic” low alloy steels.
Hydrogen atoms are the smallest of any element. As more hydrogen atoms accumulate at these areas, they combine to form relatively very large hydrogen molecules (H2) which raises internal pressure, expands the size of the defect or grain boundary interface and attracts still more hydrogen atoms, accelerating the process.
In order for hydrogen embrittlement to occur, three conditions must coincide :
- The part must have a tensile strength in excess off approximately 130,000 psi. This generally corresponds to a hardness of Rockwell C 35.
- The part must be in contact with a source of hydrogen. This may occur during manufacture, in service, or both.
- The part must be subjected to a tensile stress.
- The part must be in contact with a source of hydrogen. The part must be subjected to a tensile stress.
Residual internal stresses from casting, forging, welding and other manufacturing processes are significant and, in fact, are probably the root cause of most hydrogen embrittlement failures. Heat treating to raise strength levels above 130,000 psi induces substantial levels of residual stress. The disturbing phenomenon of “shelf popping”, unassembled parts cracking in storage or inventory with an audible “pop”, results from hydrogen embrittlement associated with residual stress.
Classification of hydrogen damage
Since the majority of the hydrogen is absorbed through and accumulates at the grain boundaries, hydrogen embrittlement cracking is primarily intergrannular (fracture at the grain boundary) rather than transgrannular (fracture through the grains) as in some other forms of brittle cracking. One of the challenges in predicting and preventing hydrogen embrittlement is the wide range of available sources of hydrogen, both in the manufacturing and service environment.
Service related sources of hydrogen may include incidental contact with acids or hydrogen containing cleaning solutions, or absorption from hydrogen containing product by equipment used in its processing. Corrosion can also act as a source of hydrogen in the manufacturing process. Rusted ingots and scrap used in casting melts, welding on parts that have corroded, and heat treating corroded parts, are potential sources of absorbed hydrogen, particularly when exposed to elevated temperatures which increase the mobility of hydrogen atoms.
While some stainless steel grades are susceptible, high strength steels with tensile strengths and hardness above 130,000 psi and Rockwell C35, respectively, are the most prone to hydrogen embrittlement. Steels below these tensile and hardness levels are generally immune. When hydrogen atoms combine into molecules in a steel that exceeds the tensile strength and hardness threshold, the steel cracks under the pressure increase. Susceptibility to hydrogen embrittlement increases in alloy steels with heat treatment to higher strength. The strength/susceptibility relationship, in fact, approaches exponential levels. In other words, doubling the heat treated strength, quadruples the steel’s susceptibility to hydrogen embrittlement. This leaves the majority of the part at “low” or undetectable hydrogen levels. Chemical analysis of parts after failure, to determine if hydrogen embrittlement is the cause, is also not viable since hydrogen diffuses from the part after fracture.
Since most hydrogen embrittlement results from hydrogen absorbed during the manufacturing process, parts which are “batch” processed are usually either all embrittled or all “good”. The two keys to avoiding hydrogen embrittlement are at the design stage and during the manufacturing process. As with other failure modalities, hydrogen absorption can inadvertently be “designed into” a part. On the manufacturing side, avoiding reducing acids where possible removes an abundant source of hydrogen from potential exposure to the part.
You might also like
| Do you know Hydrogen Embrittlement ? Hydrogen embrittlement Hydrogen embrittlement... | Hydrogen Embrittlement Hydrogen Embrittlement - Definition and Meaning When... | History of Hydrogen Discovery of Hydrogen The first recorded... | Do you know the Stress Corrosion Cracking ? Stress Corrosion Cracking Stress corrosion... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope