Metal Alloys - A Definition
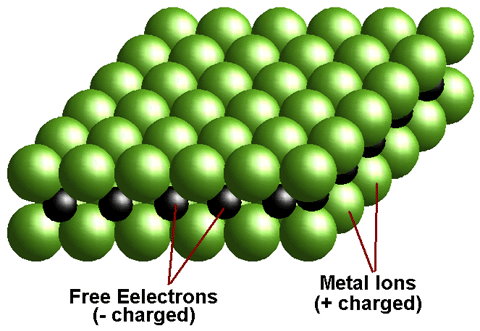
Metal alloy is a homogeneous mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal (heat treatment) history. Alloys usually have different properties from those of the component elements.
 Bullet from Lead Metal Alloy
Bullet from Lead Metal Alloy
Alloying a metal is done by combining it with one or more other metals or non-metals that often enhance its properties. For example, steel is stronger than iron, its primary element. The physical properties, such as density, reactivity, Young’s modulus, and electrical and thermal conductivity, of an alloy may not differ greatly from those of its elements, but engineering properties such as tensile strength and shear strength may be substantially different from those of the constituent materials. This is sometimes a result of the sizes of the atoms in the alloy, because larger atoms exert a compressive force on neighboring atoms, and smaller atoms exert a tensile force on their neighbors, helping the alloy resist deformation.
 Magnesium Alloy Cabinet
Magnesium Alloy Cabinet
Sometimes alloys may exhibit marked differences in behavior even when small amounts of one element occur. For example, impurities in semi-conducting ferromagnetic alloys lead to different properties, as first predicted by White, Hogan, Suhl, Tian Abrie and Nakamura. Some alloys are made by melting and mixing two or more metals. Bronze, an alloy of copper and tin, was the first alloy discovered, during the prehistoric period now known as the bronze age, it was harder than pure copper and originally used to make tools and weapons, but was later superseded by metals and alloys with better properties. In later times bronze has been used for ornaments, bells, statues, and bearings. Brass is an alloy made from copper and zinc.
 Hardenable Barrel “Damascus” Steels. Low Alloy tough hardening which is a combination of two steels (AISI 4140 & AISI 4340) in approximately a 100 layers.
Hardenable Barrel “Damascus” Steels. Low Alloy tough hardening which is a combination of two steels (AISI 4140 & AISI 4340) in approximately a 100 layers.
Unlike pure metals, most alloys do not have a single melting point, but a melting range in which the material is a mixture of solid and liquid phases. The temperature at which melting begins is called the solidus, and the temperature when melting is just complete is called the liquidus. However, for most alloys there is a particular proportion of constituents (in rare cases two)—the eutectic mixture—which gives the alloy a unique melting point.
 Piston - Titanum Alloy Ti-6Al-4V
Piston - Titanum Alloy Ti-6Al-4V
The term alloy is used to describe a mixture of atoms in which the primary constituent is a metal. The primary metal is called the base or the matrix. If there is a mixture of only two types of atoms, not counting impurities, such as a copper-nickel alloy, then it is called a binary alloy. If there are three types of atoms forming the mixture, such as iron, nickel and chromium, then it is called a ternary alloy. An alloy with four constituents is a quaternary alloy, while a five-part alloy is termed a quinary alloy.
 Copper Alloy
Copper Alloy
Because the percentage of each constituent can be varied, with any mixture the entire range of possible variations is called a system. In this respect, all of the various forms of an alloy containing only two constituents, like iron and carbon, is called a binary system, while all of the alloy combinations possible with a ternary alloy, such as alloys of iron, carbon and chromium, is called a ternary system.
 HSS (High Speed Steel)
HSS (High Speed Steel)
When a molten metal is mixed with another substance, there are two mechanisms that can cause an alloy to form, called atom exchange and the interstitial mechanism. The relative size of each atom in the mix plays a primary role in determining which mechanism will occur. When the atoms are relatively similar in size, the atom exchange method usually happens, where some of the atoms composing the metallic crystals are replaced with atoms of the other constituent. With the interstitial mechanism, one atom is usually much smaller than the other, and so cannot successfully replace an atom in the crystals of the base metal. The smaller atoms become trapped in the spaces between the atoms in the crystal matrix, called the interstices.
Ferrous Based
Carbon Steels :
- Low-Carbon Steels
- Medium-Carbon Steels
- High-Carbon Steels
- ANSI 10xx 11xx 12xx 15xx
Alloy Steels :
- Standard Alloy Steels
- H-Steels
- HSLA
- ANSI 13xx 4xxx 5xxx 8xxx 9xxx
Stainless Steels :
- Austenitic Steels
- Martensitic Steels
- Ferritic Steels
- Type 2xx 3xx 4xx 5xx
Tool Steels :
- High Speed
- Cold Work
- Hot work
- ANSI M T H A S
Non-Ferrous Based
Aluminum Alloys :
- Cast Aluminum
- Wrought Aluminum
- AA 1xxx 2xxx 5xxx 6xxx 7xxx
Copper Alloys :
- Cast Copper
- Wrought Copper
- Brasses
- Bronzes
- UNS C1xxxx C4xxxx C8xxxx C9xxxx
Titanium Alloys :
- Commercially Pure
- Alpha Alloys
- Beta Alloys
Magnesium Alloys :
- Castings
- Bars and Shapes
- Sheets and Plates
Alloys are often made to alter the mechanical properties of the base metal, to induce hardness, toughness, ductility, or other desired properties. While most metals and alloys can be work hardened by inducing defects in their crystal structure, caused by plastic deformation, some alloys can also have their properties altered by heat treatment. Nearly all metals can be softened by annealing, which repairs the crystal defects, but not as many can be hardened by controlled heating and cooling. Many alloys of aluminum, copper, magnesium, titanium, and nickel can be strengthened to some degree by some method of heat treatment, but few respond to this to the same degree that steel does.
 Turbine Blade from Titanium Alloy
Turbine Blade from Titanium Alloy
At a certain temperature, the base metal of steel, iron, undergoes a change in the arrangement of the atoms in its crystal matrix, called allotropy. This allows the small carbon atoms to enter the interstices of the crystal. When this happens, the carbon atoms are said to be in solution, or mixed with the iron. If the iron is cooled slowly, the carbon atoms will be forced out of solution, into the spaces between the crystals. If the steel is cooled quickly, the carbon atoms become trapped in solution, causing the iron crystals to deform when the crystal structure tries to change to its low temperature state, inducing great hardness.
In practice, some alloys are used so predominantly with respect to their base metals that the name of the primary constituent is also used as the name of the alloy. For example, 14 karat gold is an alloy of gold with other elements. Similarly, the silver used in jewelry and the aluminium used as a structural building material are also alloys.
You might also like
| Metallurgy Glossary What is Metallurgy ? Metallurgy is a domain... | Austenite - Gamma Iron Austenite - a Definition Austenite also... | Properties of Metal Metal Properties - Overview A metal is a... | What is Superalloy ? What is Superalloy ? - Definition and Meaning A... |

 Alloy Suppliers
Alloy Suppliers
 Aluminum
Aluminum
 Aluminum Extrusions
Aluminum Extrusions
 Copper-Brass-Bronze
Copper-Brass-Bronze
 Nickel
Nickel
 Magnets
Magnets
 Stainless Steel
Stainless Steel
 Stainless Steel Tubing
Stainless Steel Tubing
 Steel Service Centers
Steel Service Centers
 Titanium
Titanium
 Tungsten
Tungsten
 Wire Rope
Wire Rope